CHAPTER 8 Manyelectron atoms Homework due Wednesday Oct











- Slides: 22

CHAPTER 8 Many-electron atoms Homework due Wednesday Oct. 29 th: Chapter 7: 18, 20, 24, 25 Chapter 8: 1, 2, 4, 8, 10, 19 No class: Friday Oct. 24 th Wednesday Oct. 29 th Dimitri Mendeleev Turn homework into room 144: Undergraduate Office by 2: 30 pm next Wednesday

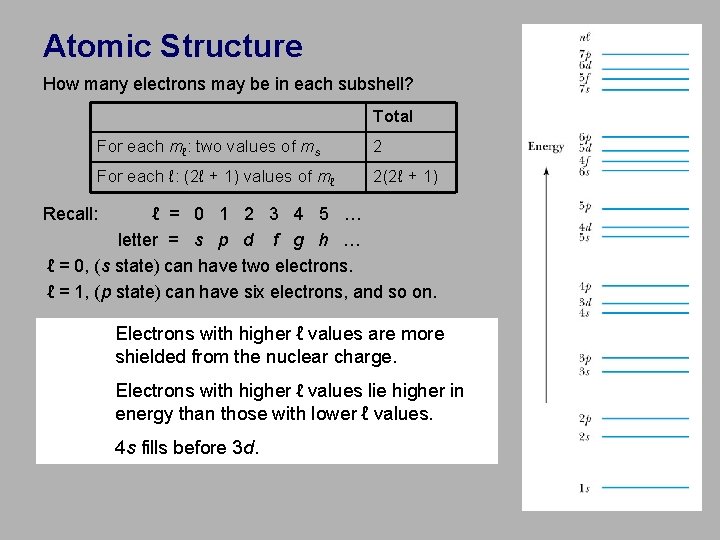
Atomic Structure How many electrons may be in each subshell? Total For each mℓ: two values of ms 2 For each ℓ: (2ℓ + 1) values of mℓ 2(2ℓ + 1) Recall: ℓ = 0 1 2 3 4 5 … letter = s p d f g h … ℓ = 0, (s state) can have two electrons. ℓ = 1, (p state) can have six electrons, and so on. Electrons with higher ℓ values are more shielded from the nuclear charge. Electrons with higher ℓ values lie higher in energy than those with lower ℓ values. 4 s fills before 3 d.

The Periodic Table

Inert Gases: Last group of the periodic table Closed p subshell except helium Zero net spin and large ionization energy Their atoms interact weakly with each other Alkalis: Single s electron outside an inner core Easily form positive ions with a charge +1 e Lowest ionization energies Electrical conductivity is relatively good Alkaline Earths: Two s electrons in outer subshell Largest atomic radii High electrical conductivity The Periodic Table

The Periodic Table Halogens: Need one more electron to fill outermost subshell Form strong ionic bonds with the alkalis More stable configurations occur as the p subshell is filled Transition Metals: Three rows of elements in which the 3 d, 4 d, and 5 d are being filled Properties primarily determined by the s electrons, rather than by the d subshell being filled Have d-shell electrons with unpaired spins As the d subshell is filled, the magnetic moments, and the tendency for neighboring atoms to align spins are reduced

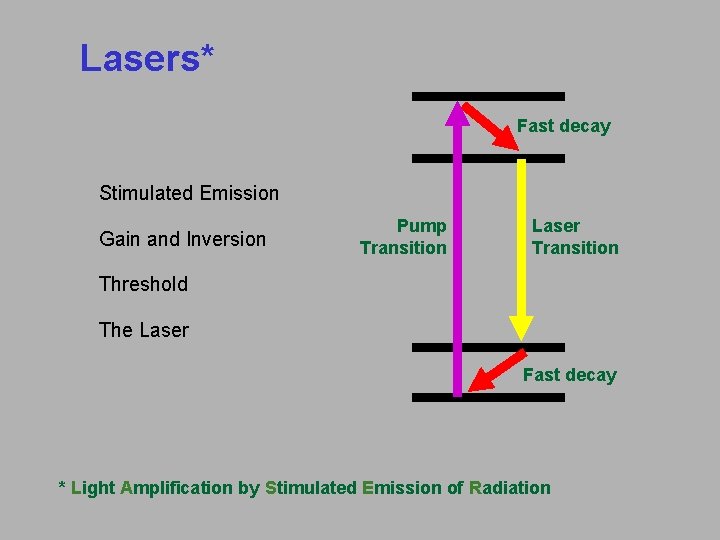
Lasers* Fast decay Stimulated Emission Gain and Inversion Pump Transition Laser Transition Threshold The Laser Fast decay * Light Amplification by Stimulated Emission of Radiation

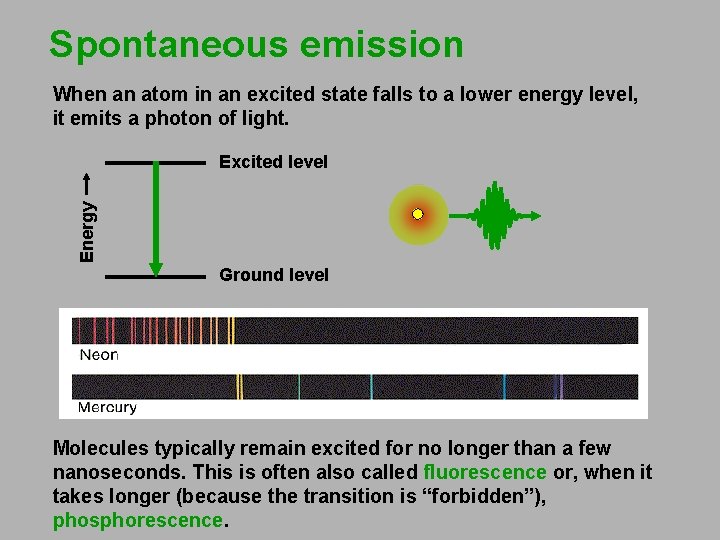
Spontaneous emission When an atom in an excited state falls to a lower energy level, it emits a photon of light. Energy Excited level Ground level Molecules typically remain excited for no longer than a few nanoseconds. This is often also called fluorescence or, when it takes longer (because the transition is “forbidden”), phosphorescence.

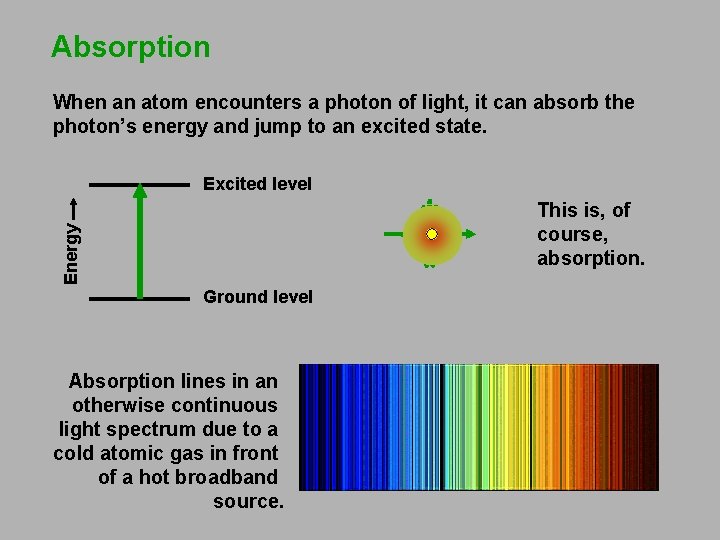
Absorption When an atom encounters a photon of light, it can absorb the photon’s energy and jump to an excited state. Excited level Energy This is, of course, absorption. Ground level Absorption lines in an otherwise continuous light spectrum due to a cold atomic gas in front of a hot broadband source.

Einstein showed that another process, stimulated emission, can also occur. When a photon encounters an atom in an excited state, the photon can induce the atom to emit its energy as another photon of light, resulting in two identical photons. Energy Excited level Ground level Einstein first proposed stimulated emission in 1916.

In what energy levels do molecules reside? Boltzmann Population Factors Energy E 3 E 2 E 1 Ni is the number density (also known as the population density) of molecules in state i (i. e. , the number of molecules per cm 3). N 3 N 2 N 1 Population density (Number of molecules per unit volume) T is the temperature, and k. B is Boltzmann’s constant = 1. 3806503 × 10 -23 J/K

The Maxwell-Boltzman distribution Collisions can knock a molecule into a higher-energy state. The higher the temperature, the more this happens. Energy Low T High T 3 2 Molecules Energy In the absence of collisions, molecules tend to remain in the lowest energy state available. Molecules 1 1 The ratio of the population densities of two states is: N 2 / N 1 = exp(–DE/k. BT ), where DE = E 2 – E 1 = hn As a result, higher-energy states are always less populated than the ground state, and absorption is stronger than stimulated emission.

Stimulated emission leads to a chain reaction and laser emission. If a medium has many excited molecules, one photon can become many. Excited medium This is the essence of the laser. The factor by which an input beam is amplified by a medium is called the gain and is represented by G.

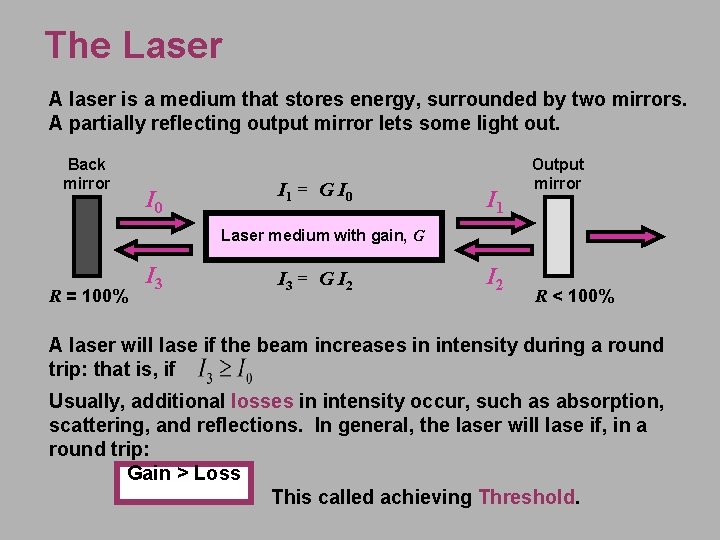
The Laser A laser is a medium that stores energy, surrounded by two mirrors. A partially reflecting output mirror lets some light out. Back mirror I 0 I 1 = G I 0 I 1 Output mirror Laser medium with gain, G R = 100% I 3 = G I 2 R < 100% A laser will lase if the beam increases in intensity during a round trip: that is, if Usually, additional losses in intensity occur, such as absorption, scattering, and reflections. In general, the laser will lase if, in a round trip: Gain > Loss This called achieving Threshold.

Inversion In order to achieve G > 1, stimulated emission must exceed absorption: B N 2 I > B N 1 I Inversion N 2 > N 1 This condition is called inversion. It does not occur naturally (it’s forbidden by the Boltzmann distribution). It’s inherently a non-equilibrium state. Energy Canceling the BI factors, 4 3 2 “Negative temperature ” Molecules 1 Here, there is inversion from level 4 to levels 3 and 2. In order to achieve inversion, we must hit the laser medium very hard in some way and choose our medium correctly.

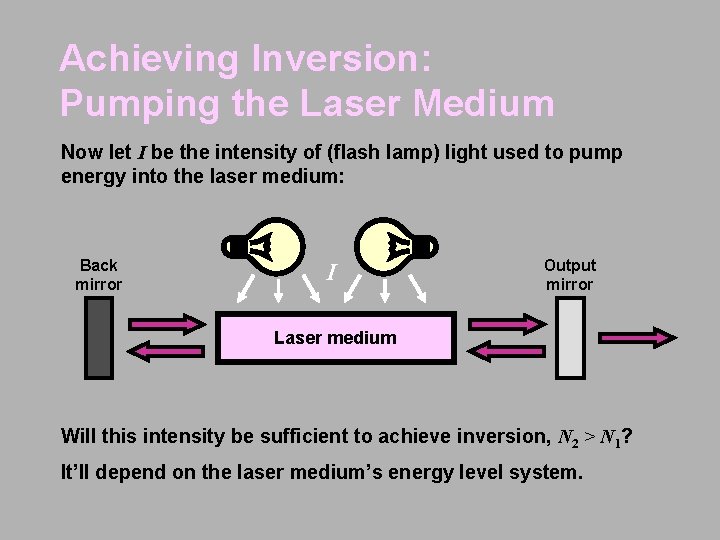
Achieving Inversion: Pumping the Laser Medium Now let I be the intensity of (flash lamp) light used to pump energy into the laser medium: Back mirror I Output mirror Laser medium Will this intensity be sufficient to achieve inversion, N 2 > N 1? It’ll depend on the laser medium’s energy level system.

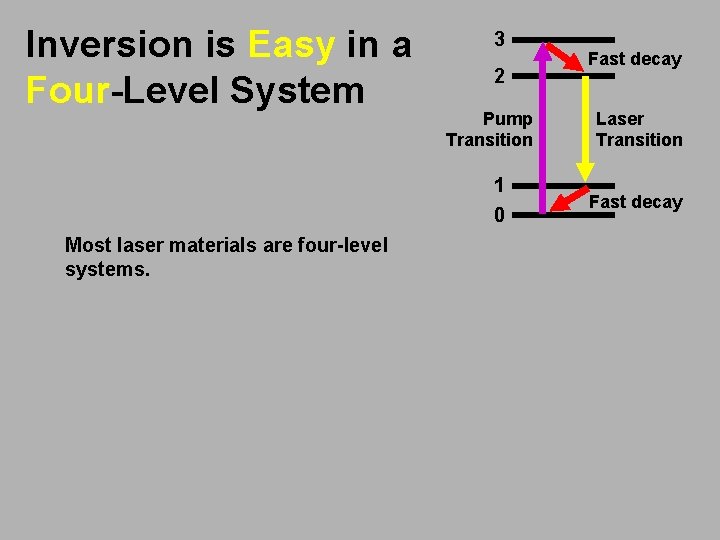
Inversion is Easy in a Four-Level System 3 2 Pump Transition 1 0 Most laser materials are four-level systems. Fast decay Laser Transition Fast decay

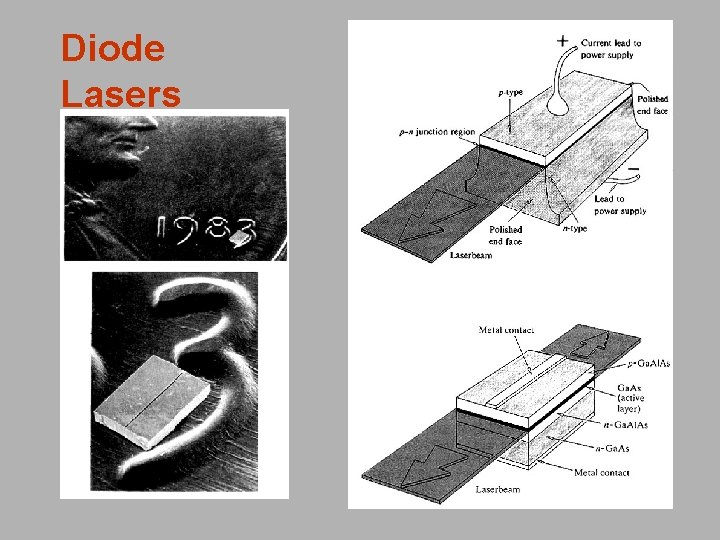
Types of Lasers Solid-state lasers have lasing material distributed in a solid matrix (such as ruby or neodymium: yttrium-aluminum garnet "YAG"). Flash lamps are the most common power source. The Nd: YAG laser emits infrared light at 1, 064 nm (1. 064 mm). Semiconductor lasers, sometimes called diode lasers, are pn junctions. Current is the pump source. Applications: laser printers or CD players. Dye lasers use complex organic dyes, such as rhodamine 6 G, in liquid solution or suspension as lasing media. They are tunable over a broad range of wavelengths. Gas lasers are pumped by current. Helium-Neon lases in the visible and IR. Argon lases in the visible and UV. CO 2 lasers emit light in the far-infrared (10. 6 mm), and are used for cutting hard materials. Excimer lasers (from the terms excited and dimers) use reactive gases, such as chlorine and fluorine, mixed with inert gases such as argon, krypton, or xenon. When electrically stimulated, a pseudo molecule (dimer) is produced. Excimers lase in the UV.

Diode Lasers

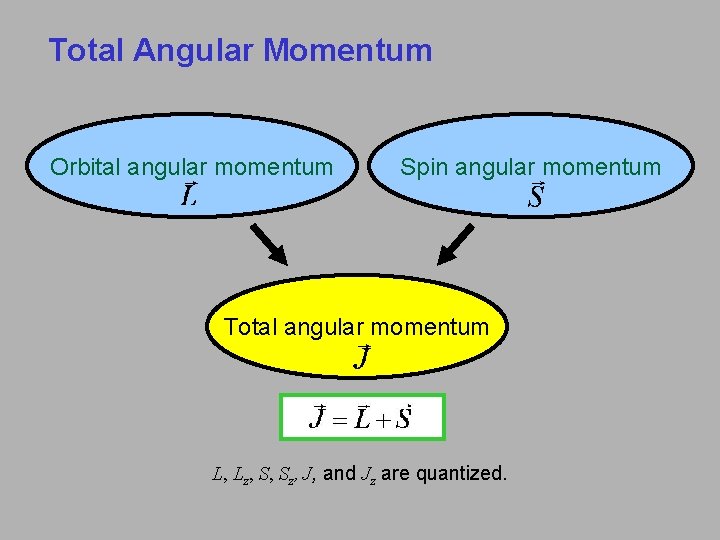
Total Angular Momentum Orbital angular momentum Spin angular momentum Total angular momentum L, Lz, S, Sz, J, and Jz are quantized.

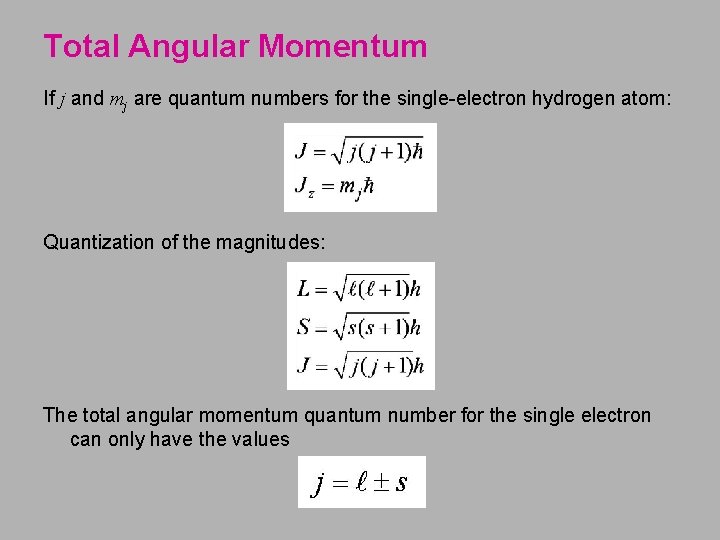
Total Angular Momentum If j and mj are quantum numbers for the single-electron hydrogen atom: Quantization of the magnitudes: The total angular momentum quantum number for the single electron can only have the values

Spin-Orbit Coupling An effect of the spins of the electron and the orbital angular momentum interaction is called spin-orbit coupling. The dipole potential energy The spin magnetic moment µ is the magnetic field due to the electron’s orbital motion. ● where a is the angle between .

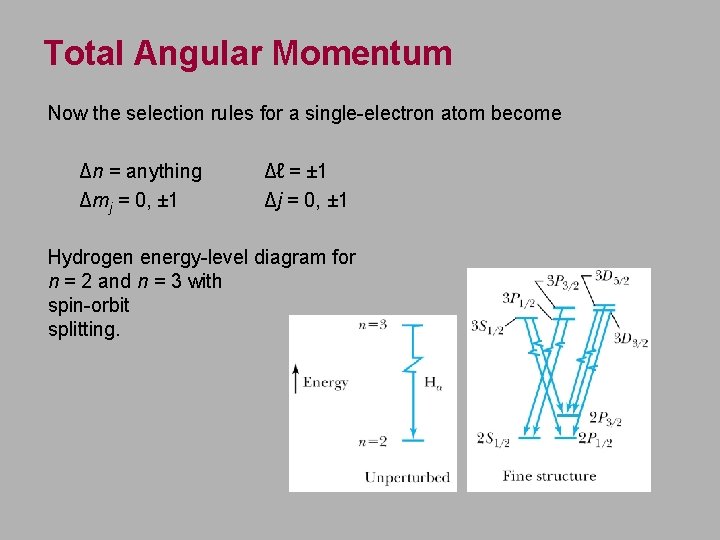
Total Angular Momentum Now the selection rules for a single-electron atom become Δn = anything Δmj = 0, ± 1 Δℓ = ± 1 Δj = 0, ± 1 Hydrogen energy-level diagram for n = 2 and n = 3 with spin-orbit splitting.