CHAPTER 8 Manyelectron atoms Homework next Friday Oct





- Slides: 18

CHAPTER 8 Many-electron atoms Homework next Friday Oct. 30 th: Chapter 7: 18, 20, 24, 25 Chapter 8: 1, 2, 4, 8, 10, 19 Dimitri Mendeleev What distinguished Mendeleev was not only genius, but a passion for the elements. They became his personal friends; he knew every quirk and detail of their behavior. - J. Bronowski

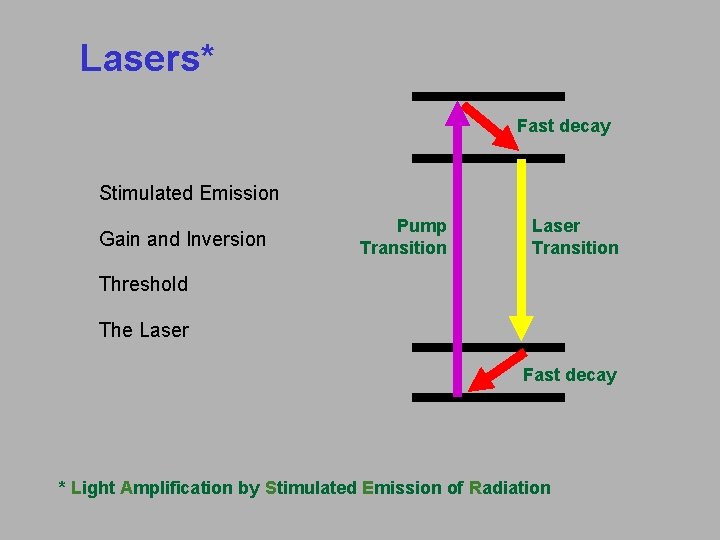
Lasers* Fast decay Stimulated Emission Gain and Inversion Pump Transition Laser Transition Threshold The Laser Fast decay * Light Amplification by Stimulated Emission of Radiation

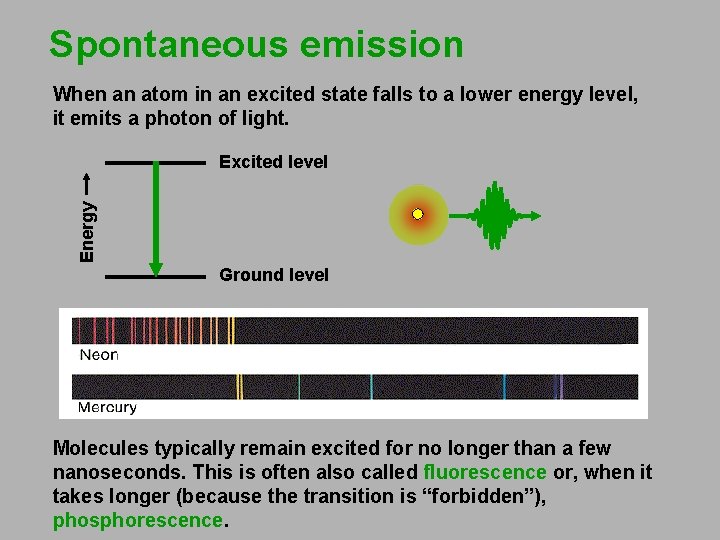
Spontaneous emission When an atom in an excited state falls to a lower energy level, it emits a photon of light. Energy Excited level Ground level Molecules typically remain excited for no longer than a few nanoseconds. This is often also called fluorescence or, when it takes longer (because the transition is “forbidden”), phosphorescence.

Absorption When an atom encounters a photon of light, it can absorb the photon’s energy and jump to an excited state. Excited level Energy This is, of course, absorption. Ground level Absorption lines in an otherwise continuous light spectrum due to a cold atomic gas in front of a hot broadband source.

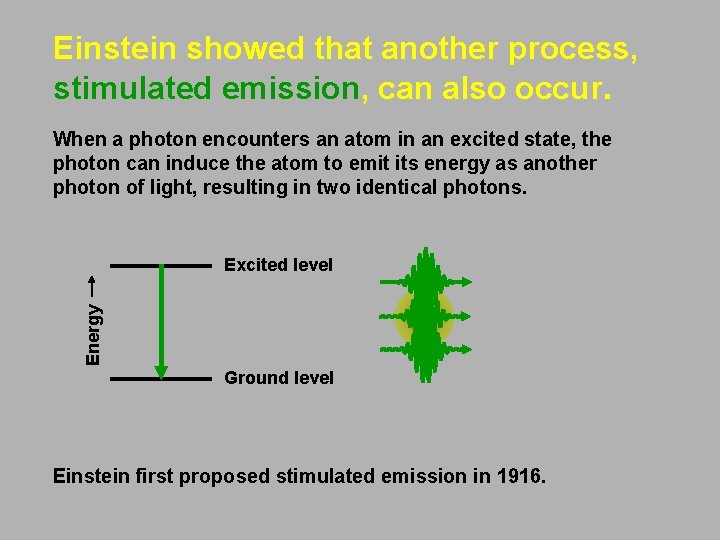
Einstein showed that another process, stimulated emission, can also occur. When a photon encounters an atom in an excited state, the photon can induce the atom to emit its energy as another photon of light, resulting in two identical photons. Energy Excited level Ground level Einstein first proposed stimulated emission in 1916.

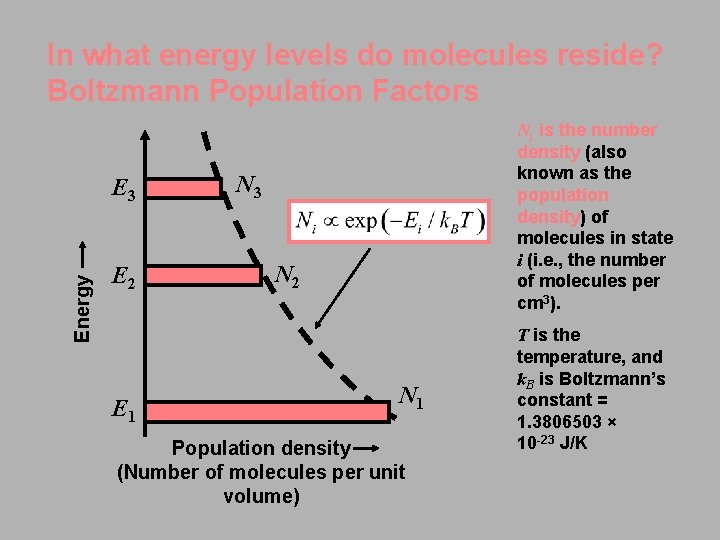
In what energy levels do molecules reside? Boltzmann Population Factors Energy E 3 E 2 E 1 Ni is the number density (also known as the population density) of molecules in state i (i. e. , the number of molecules per cm 3). N 3 N 2 N 1 Population density (Number of molecules per unit volume) T is the temperature, and k. B is Boltzmann’s constant = 1. 3806503 × 10 -23 J/K

The Maxwell-Boltzman distribution Collisions can knock a molecule into a higher-energy state. The higher the temperature, the more this happens. Energy Low T High T 3 2 Molecules Energy In the absence of collisions, molecules tend to remain in the lowest energy state available. Molecules 1 1 The ratio of the population densities of two states is: N 2 / N 1 = exp(–DE/k. BT ), where DE = E 2 – E 1 = hn As a result, higher-energy states are always less populated than the ground state, and absorption is stronger than stimulated emission.

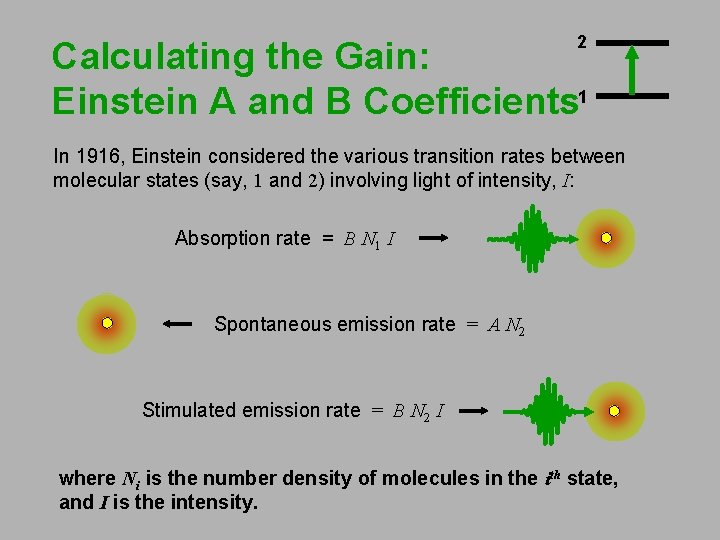
2 Calculating the Gain: Einstein A and B Coefficients 1 In 1916, Einstein considered the various transition rates between molecular states (say, 1 and 2) involving light of intensity, I: Absorption rate = B N 1 I Spontaneous emission rate = A N 2 Stimulated emission rate = B N 2 I where Ni is the number density of molecules in the ith state, and I is the intensity.

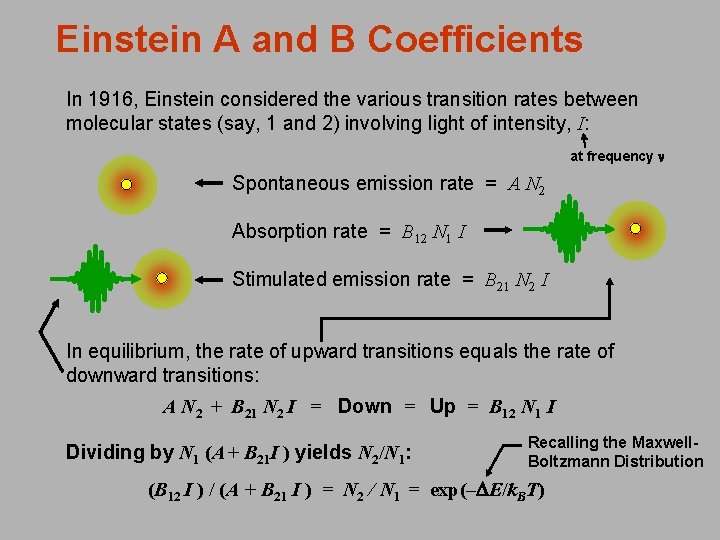
Einstein A and B Coefficients In 1916, Einstein considered the various transition rates between molecular states (say, 1 and 2) involving light of intensity, I: at frequency n Spontaneous emission rate = A N 2 Absorption rate = B 12 N 1 I Stimulated emission rate = B 21 N 2 I In equilibrium, the rate of upward transitions equals the rate of downward transitions: A N 2 + B 21 N 2 I = Down = Up = B 12 N 1 I Dividing by N 1 (A + B 21 I ) yields N 2/N 1: Recalling the Maxwell. Boltzmann Distribution (B 12 I ) / (A + B 21 I ) = N 2 / N 1 = exp(–DE/k. BT)

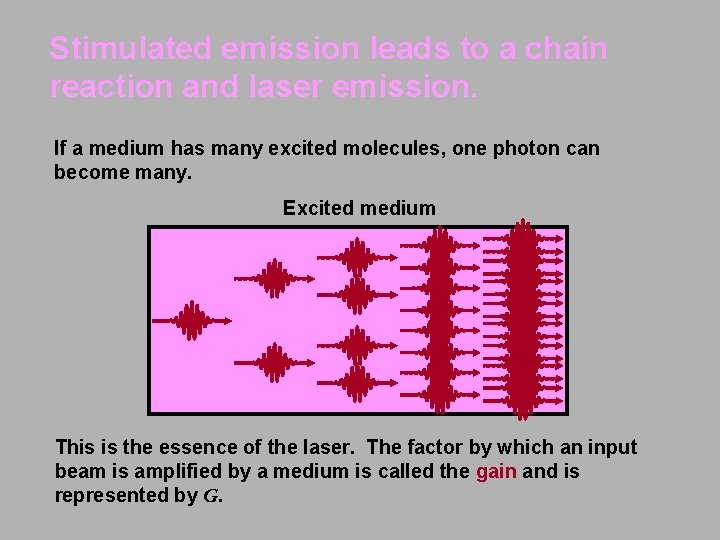
Stimulated emission leads to a chain reaction and laser emission. If a medium has many excited molecules, one photon can become many. Excited medium This is the essence of the laser. The factor by which an input beam is amplified by a medium is called the gain and is represented by G.

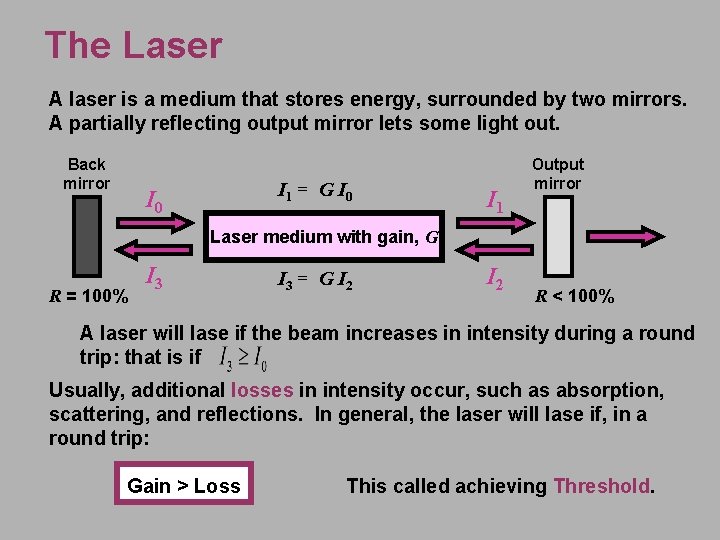
The Laser A laser is a medium that stores energy, surrounded by two mirrors. A partially reflecting output mirror lets some light out. Back mirror I 0 I 1 = G I 0 I 1 Output mirror Laser medium with gain, G R = 100% I 3 = G I 2 R < 100% A laser will lase if the beam increases in intensity during a round trip: that is if Usually, additional losses in intensity occur, such as absorption, scattering, and reflections. In general, the laser will lase if, in a round trip: Gain > Loss This called achieving Threshold.

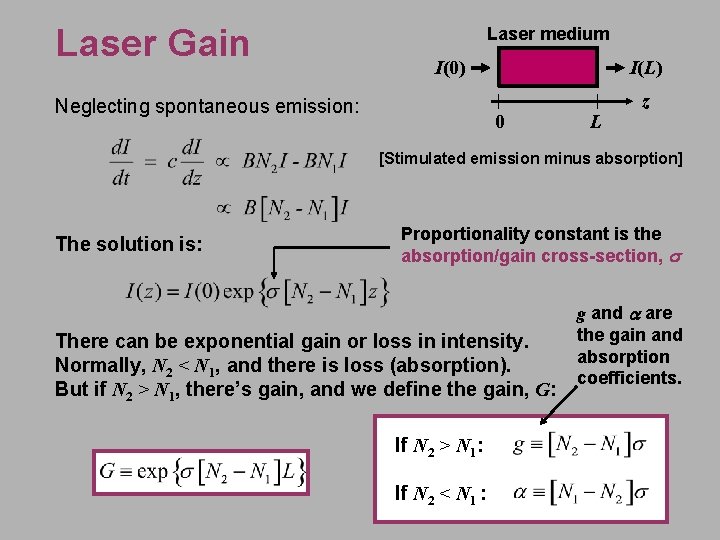
Laser Gain Laser medium I(0) Neglecting spontaneous emission: I(L) 0 L z [Stimulated emission minus absorption] The solution is: Proportionality constant is the absorption/gain cross-section, s There can be exponential gain or loss in intensity. Normally, N 2 < N 1, and there is loss (absorption). But if N 2 > N 1, there’s gain, and we define the gain, G: If N 2 > N 1: If N 2 < N 1 : g and a are the gain and absorption coefficients.

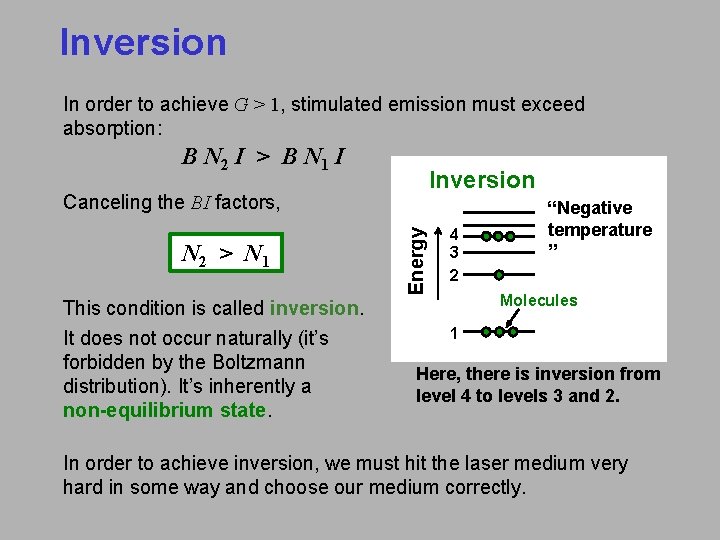
Inversion In order to achieve G > 1, stimulated emission must exceed absorption: B N 2 I > B N 1 I Inversion N 2 > N 1 This condition is called inversion. It does not occur naturally (it’s forbidden by the Boltzmann distribution). It’s inherently a non-equilibrium state. Energy Canceling the BI factors, 4 3 2 “Negative temperature ” Molecules 1 Here, there is inversion from level 4 to levels 3 and 2. In order to achieve inversion, we must hit the laser medium very hard in some way and choose our medium correctly.

Achieving Inversion: Pumping the Laser Medium Now let I be the intensity of (flash lamp) light used to pump energy into the laser medium: Back mirror I Output mirror Laser medium Will this intensity be sufficient to achieve inversion, N 2 > N 1? It’ll depend on the laser medium’s energy level system.

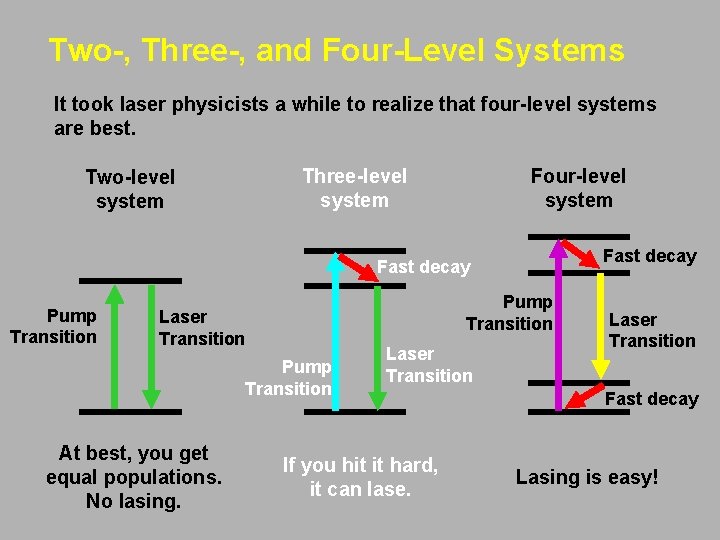
Two-, Three-, and Four-Level Systems It took laser physicists a while to realize that four-level systems are best. Four-level system Three-level system Two-level system Fast decay Pump Transition Laser Transition Pump Transition At best, you get equal populations. No lasing. Laser Transition If you hit it hard, it can lase. Laser Transition Fast decay Lasing is easy!

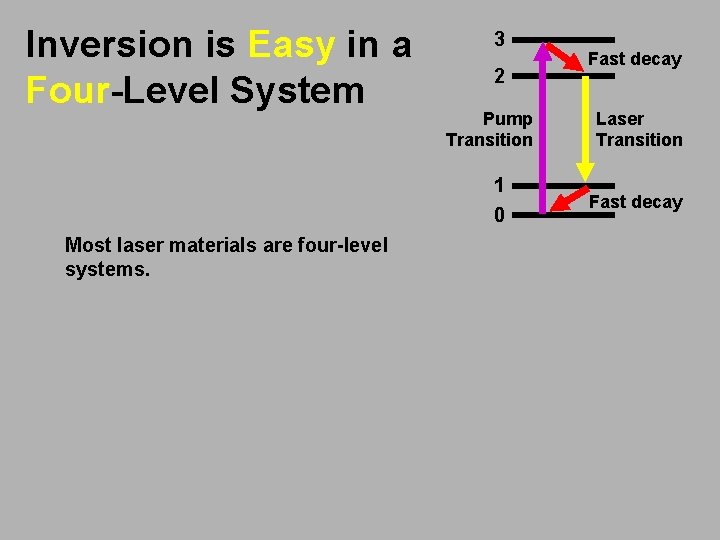
Inversion is Easy in a Four-Level System 3 2 Pump Transition 1 0 Most laser materials are four-level systems. Fast decay Laser Transition Fast decay

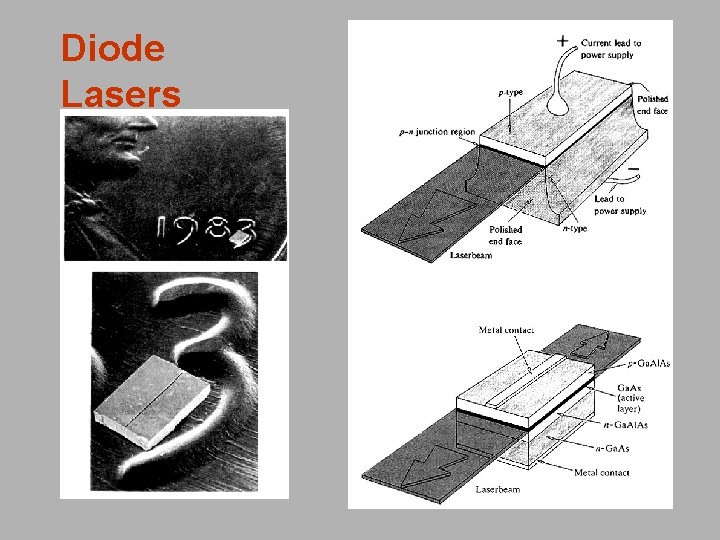
Types of Lasers Solid-state lasers have lasing material distributed in a solid matrix (such as ruby or neodymium: yttrium-aluminum garnet "YAG"). Flash lamps are the most common power source. The Nd: YAG laser emits infrared light at 1, 064 nm (1. 064 mm). Semiconductor lasers, sometimes called diode lasers, are pn junctions. Current is the pump source. Applications: laser printers or CD players. Dye lasers use complex organic dyes, such as rhodamine 6 G, in liquid solution or suspension as lasing media. They are tunable over a broad range of wavelengths. Gas lasers are pumped by current. Helium-Neon lases in the visible and IR. Argon lases in the visible and UV. CO 2 lasers emit light in the far-infrared (10. 6 mm), and are used for cutting hard materials. Excimer lasers (from the terms excited and dimers) use reactive gases, such as chlorine and fluorine, mixed with inert gases such as argon, krypton, or xenon. When electrically stimulated, a pseudo molecule (dimer) is produced. Excimers lase in the UV.

Diode Lasers