Chapter 8 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical





- Slides: 40

Chapter 8 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Chapter 8 The Mole Concept by Christopher G. Hamaker Illinois State University © 2014 Pearson Education, Inc.

Avogadro’s Number • Avogadro’s number (symbol N) is the number of atoms in 12. 01 grams of carbon. • Its numerical value is 6. 02 x 1023. • Therefore, a 12. 01 g sample of carbon contains 6. 02 x 1023 carbon atoms. © 2014 Pearson Education, Inc. Chapter 8

Analogies for Avogadro’s Number • The volume occupied by one mole of softballs would be about the size of Earth. • One mole of Olympic shot put balls has about the same mass as that of Earth. • One mole of hydrogen atoms laid side by side would circle Earth about 1 million times. © 2014 Pearson Education, Inc. Chapter 8

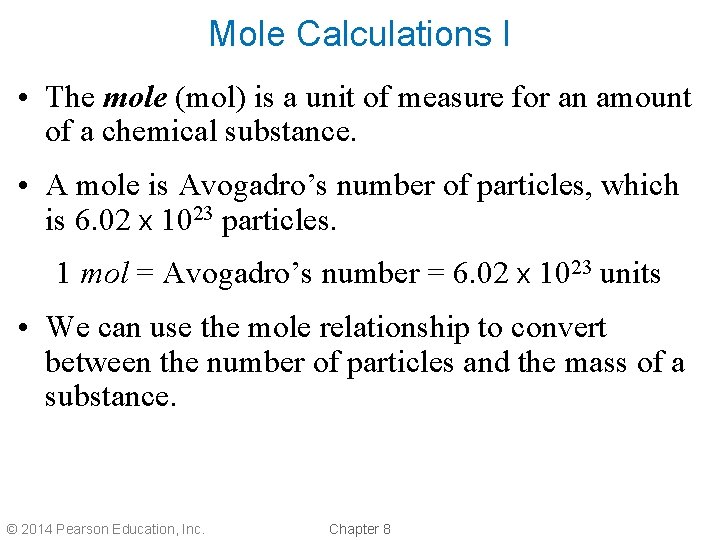
Mole Calculations I • The mole (mol) is a unit of measure for an amount of a chemical substance. • A mole is Avogadro’s number of particles, which is 6. 02 x 1023 particles. 1 mol = Avogadro’s number = 6. 02 x 1023 units • We can use the mole relationship to convert between the number of particles and the mass of a substance. © 2014 Pearson Education, Inc. Chapter 8

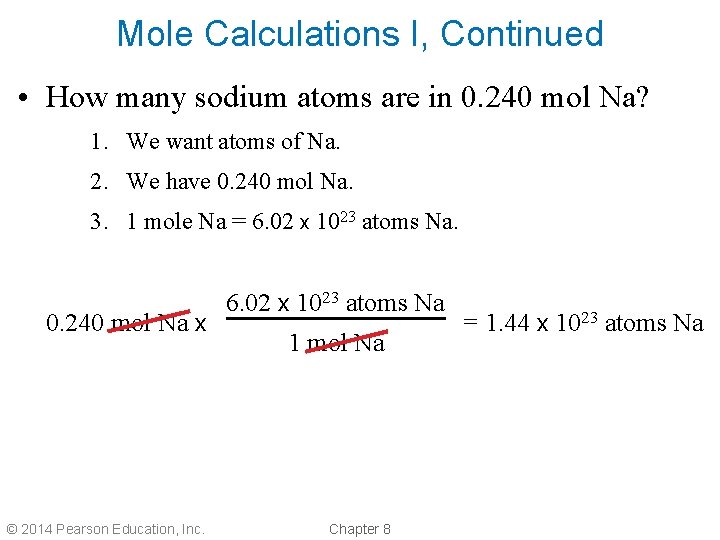
Mole Calculations I, Continued • How many sodium atoms are in 0. 240 mol Na? 1. We want atoms of Na. 2. We have 0. 240 mol Na. 3. 1 mole Na = 6. 02 x 1023 atoms Na. 0. 240 mol Na x © 2014 Pearson Education, Inc. 6. 02 x 1023 atoms Na 1 mol Na Chapter 8 = 1. 44 x 1023 atoms Na

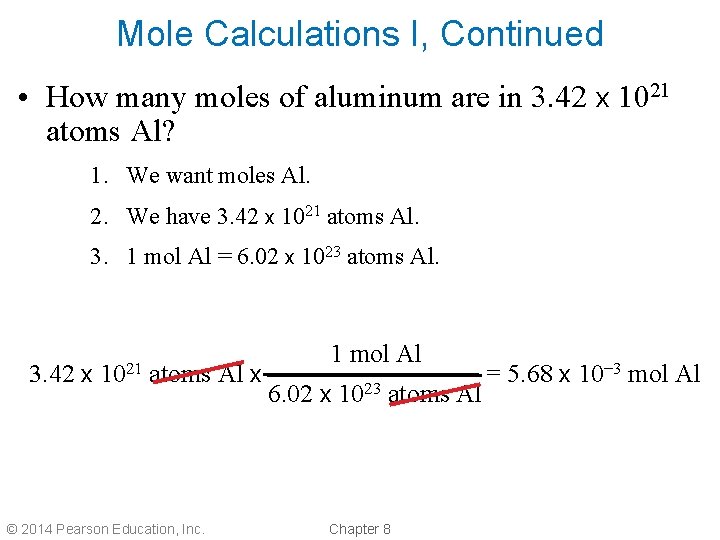
Mole Calculations I, Continued • How many moles of aluminum are in 3. 42 x 1021 atoms Al? 1. We want moles Al. 2. We have 3. 42 x 1021 atoms Al. 3. 1 mol Al = 6. 02 x 1023 atoms Al. 3. 42 x 1021 atoms Al x © 2014 Pearson Education, Inc. 1 mol Al 6. 02 x 1023 atoms Al Chapter 8 = 5. 68 x 10– 3 mol Al

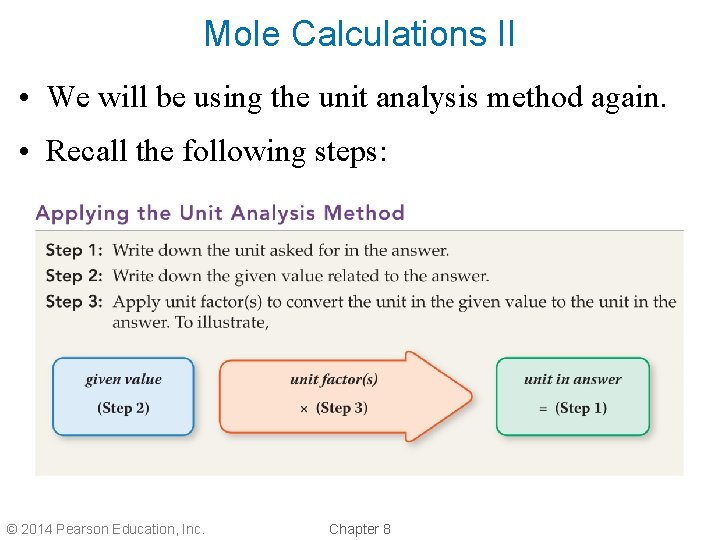
Mole Calculations II • We will be using the unit analysis method again. • Recall the following steps: © 2014 Pearson Education, Inc. Chapter 8

Molar Mass • The atomic mass of any substance expressed in grams is the molar mass (MM) of that substance. • The atomic mass of carbon is 12. 01 amu. • Therefore, the molar mass of carbon is 12. 01 g/mol. • Since nitrogen occurs naturally as a diatomic, N 2, the molar mass of nitrogen gas is two times 14. 01 g or 28. 02 g/mol. © 2014 Pearson Education, Inc. Chapter 8

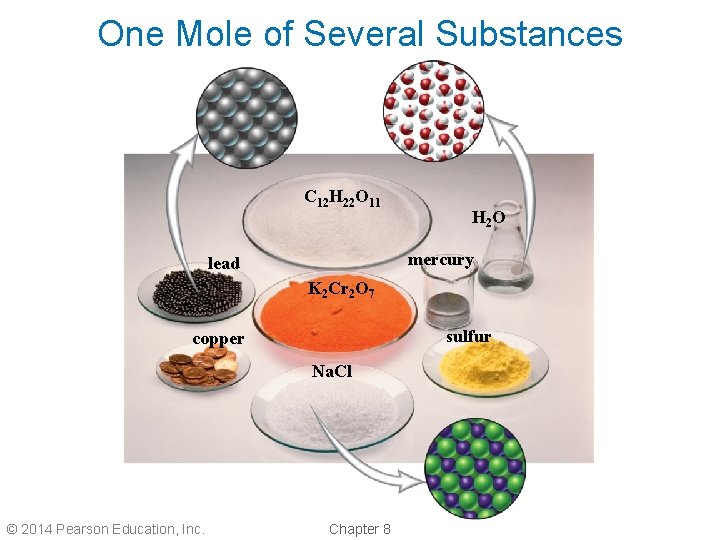
One Mole of Several Substances C 12 H 22 O 11 H 2 O mercury lead K 2 Cr 2 O 7 sulfur copper Na. Cl © 2014 Pearson Education, Inc. Chapter 8

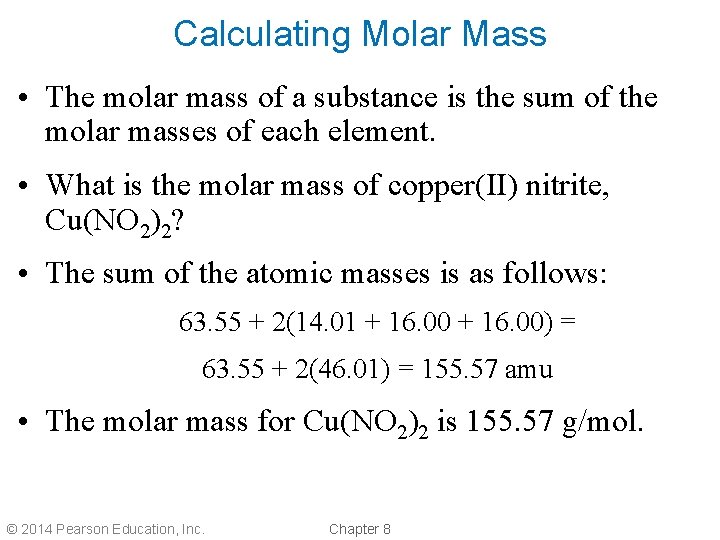
Calculating Molar Mass • The molar mass of a substance is the sum of the molar masses of each element. • What is the molar mass of copper(II) nitrite, Cu(NO 2)2? • The sum of the atomic masses is as follows: 63. 55 + 2(14. 01 + 16. 00) = 63. 55 + 2(46. 01) = 155. 57 amu • The molar mass for Cu(NO 2)2 is 155. 57 g/mol. © 2014 Pearson Education, Inc. Chapter 8

Mole Calculations II • Now we will use the molar mass of a compound to convert between grams of a substance and moles or particles of a substance. 6. 02 x 1023 particles = 1 mol = molar mass • If we want to convert particles to mass, we must first convert particles to moles, and then we can convert moles to mass. © 2014 Pearson Education, Inc. Chapter 8

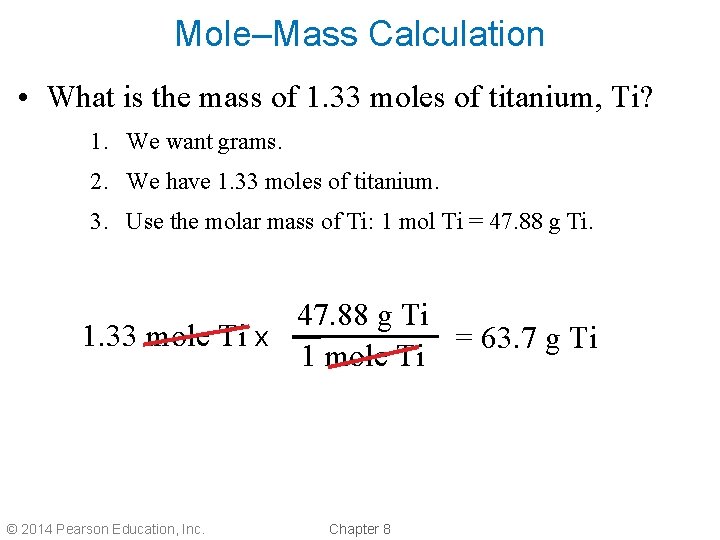
Mole–Mass Calculation • What is the mass of 1. 33 moles of titanium, Ti? 1. We want grams. 2. We have 1. 33 moles of titanium. 3. Use the molar mass of Ti: 1 mol Ti = 47. 88 g Ti 1. 33 mole Ti x = 63. 7 g Ti 1 mole Ti © 2014 Pearson Education, Inc. Chapter 8

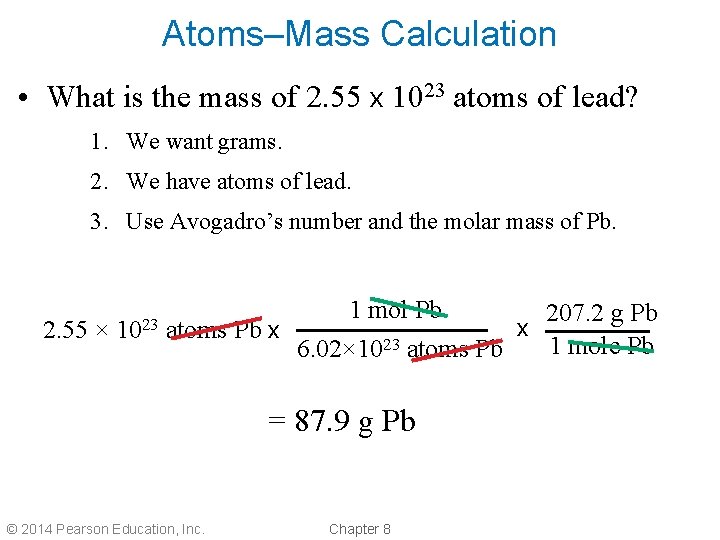
Atoms–Mass Calculation • What is the mass of 2. 55 x 1023 atoms of lead? 1. We want grams. 2. We have atoms of lead. 3. Use Avogadro’s number and the molar mass of Pb. 2. 55 × 1023 atoms Pb x 1 mol Pb 6. 02× 1023 atoms Pb = 87. 9 g Pb © 2014 Pearson Education, Inc. Chapter 8 x 207. 2 g Pb 1 mole Pb

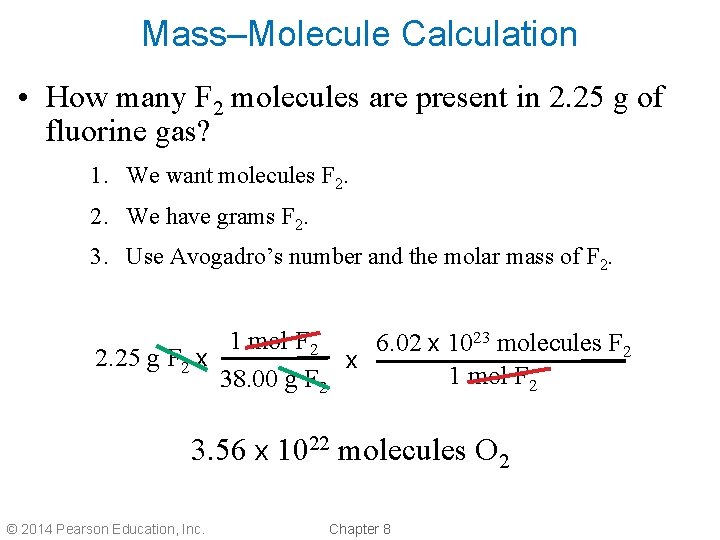
Mass–Molecule Calculation • How many F 2 molecules are present in 2. 25 g of fluorine gas? 1. We want molecules F 2. 2. We have grams F 2. 3. Use Avogadro’s number and the molar mass of F 2. 1 mol F 2 6. 02 x 1023 molecules F 2 2. 25 g F 2 x x 1 mol F 2 38. 00 g F 2 3. 56 x 1022 molecules O 2 © 2014 Pearson Education, Inc. Chapter 8

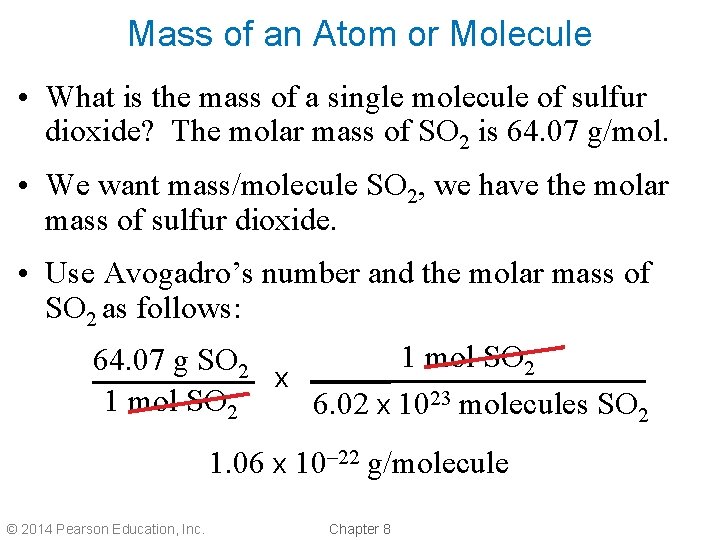
Mass of an Atom or Molecule • What is the mass of a single molecule of sulfur dioxide? The molar mass of SO 2 is 64. 07 g/mol. • We want mass/molecule SO 2, we have the molar mass of sulfur dioxide. • Use Avogadro’s number and the molar mass of SO 2 as follows: 1 mol SO 2 64. 07 g SO 2 x 1 mol SO 2 6. 02 x 1023 molecules SO 2 1. 06 x 10– 22 g/molecule © 2014 Pearson Education, Inc. Chapter 8

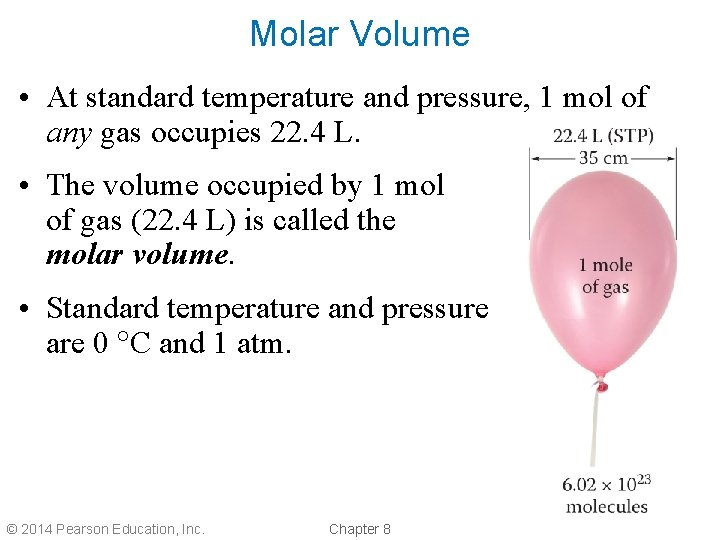
Molar Volume • At standard temperature and pressure, 1 mol of any gas occupies 22. 4 L. • The volume occupied by 1 mol of gas (22. 4 L) is called the molar volume. • Standard temperature and pressure are 0 C and 1 atm. © 2014 Pearson Education, Inc. Chapter 8

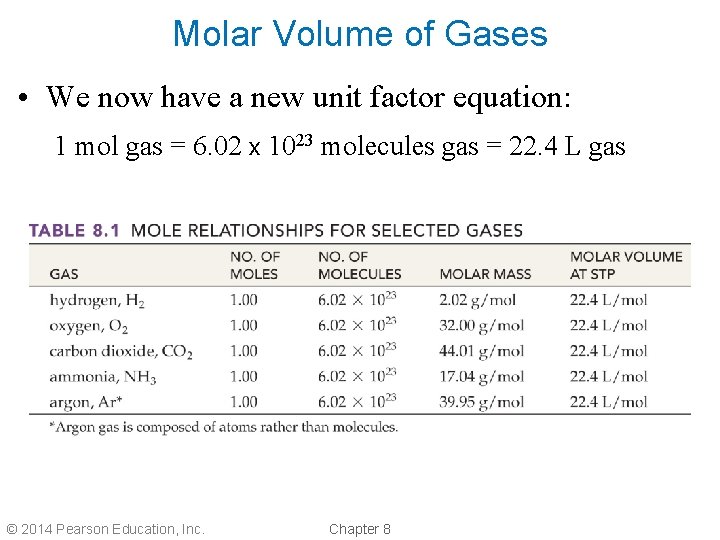
Molar Volume of Gases • We now have a new unit factor equation: 1 mol gas = 6. 02 x 1023 molecules gas = 22. 4 L gas © 2014 Pearson Education, Inc. Chapter 8

One Mole of a Gas at STP • The box below has a volume of 22. 4 L, which is the volume occupied by 1 mol of a gas at STP. © 2014 Pearson Education, Inc. Chapter 8

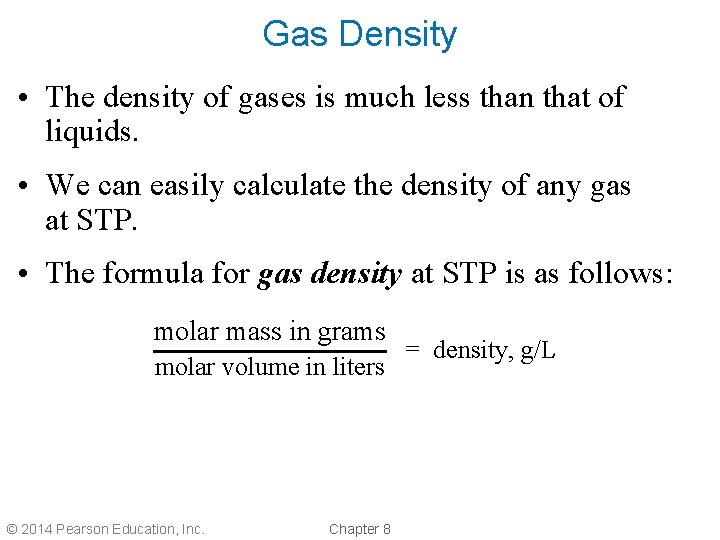
Gas Density • The density of gases is much less than that of liquids. • We can easily calculate the density of any gas at STP. • The formula for gas density at STP is as follows: molar mass in grams molar volume in liters © 2014 Pearson Education, Inc. Chapter 8 = density, g/L

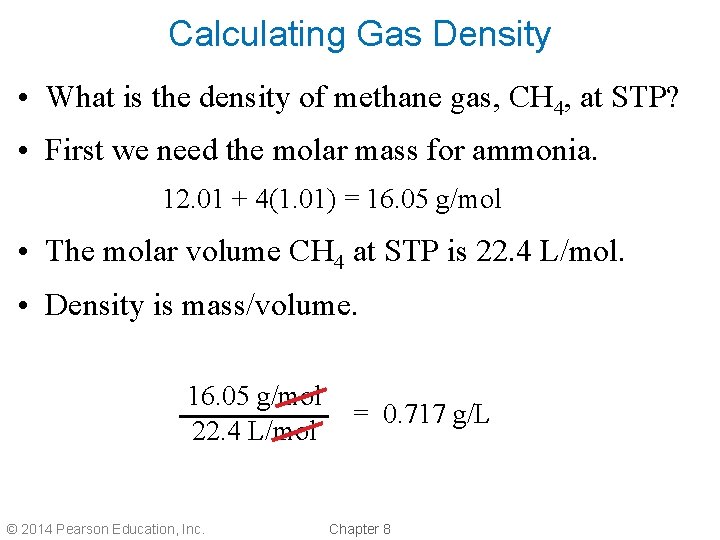
Calculating Gas Density • What is the density of methane gas, CH 4, at STP? • First we need the molar mass for ammonia. 12. 01 + 4(1. 01) = 16. 05 g/mol • The molar volume CH 4 at STP is 22. 4 L/mol. • Density is mass/volume. 16. 05 g/mol 22. 4 L/mol © 2014 Pearson Education, Inc. = 0. 717 g/L Chapter 8

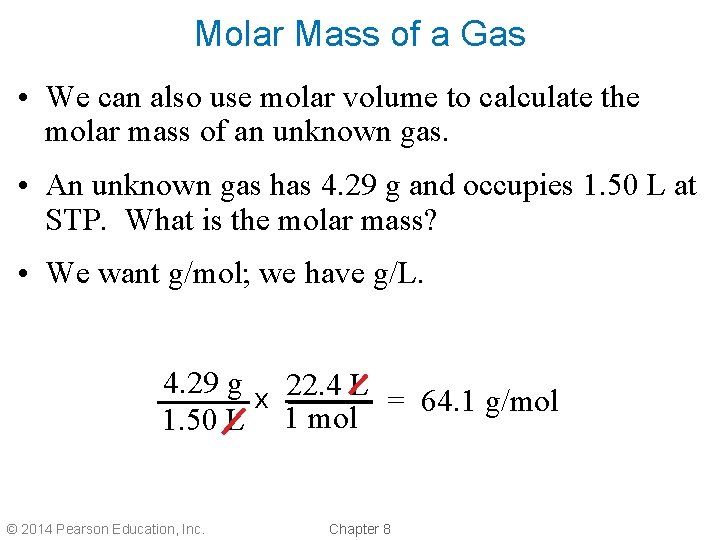
Molar Mass of a Gas • We can also use molar volume to calculate the molar mass of an unknown gas. • An unknown gas has 4. 29 g and occupies 1. 50 L at STP. What is the molar mass? • We want g/mol; we have g/L. 4. 29 g x 22. 4 L = 64. 1 g/mol 1. 50 L 1 mol © 2014 Pearson Education, Inc. Chapter 8

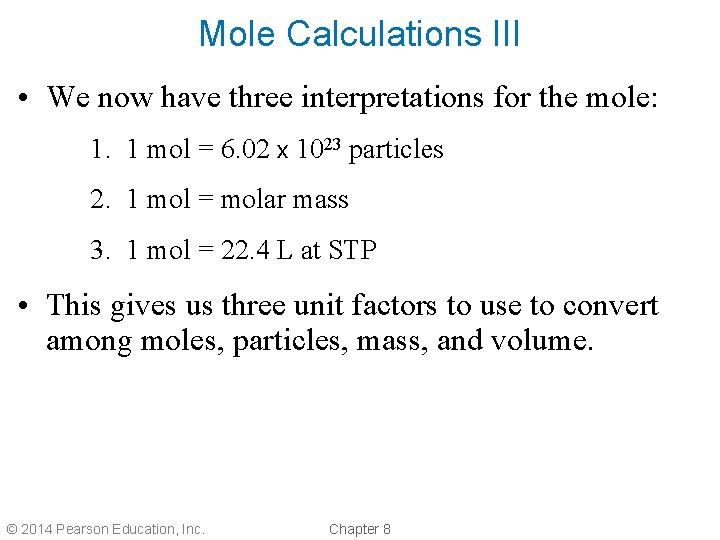
Mole Calculations III • We now have three interpretations for the mole: 1. 1 mol = 6. 02 x 1023 particles 2. 1 mol = molar mass 3. 1 mol = 22. 4 L at STP • This gives us three unit factors to use to convert among moles, particles, mass, and volume. © 2014 Pearson Education, Inc. Chapter 8

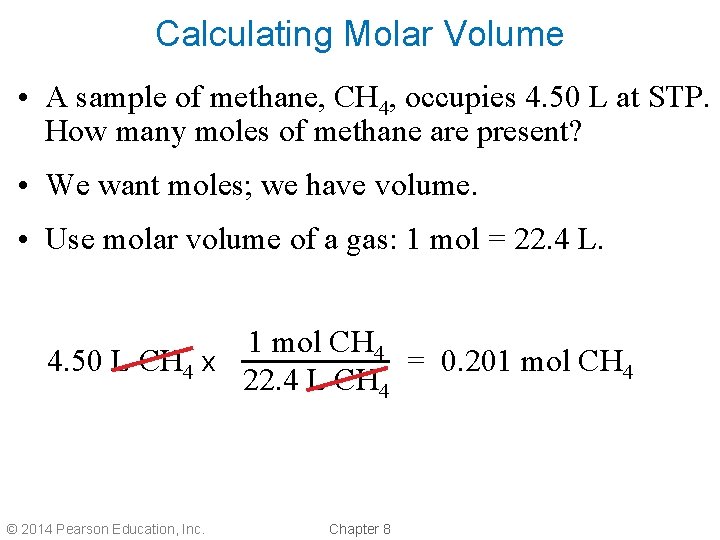
Calculating Molar Volume • A sample of methane, CH 4, occupies 4. 50 L at STP. How many moles of methane are present? • We want moles; we have volume. • Use molar volume of a gas: 1 mol = 22. 4 L. 1 mol CH 4 4. 50 L CH 4 x = 0. 201 mol CH 4 22. 4 L CH 4 © 2014 Pearson Education, Inc. Chapter 8

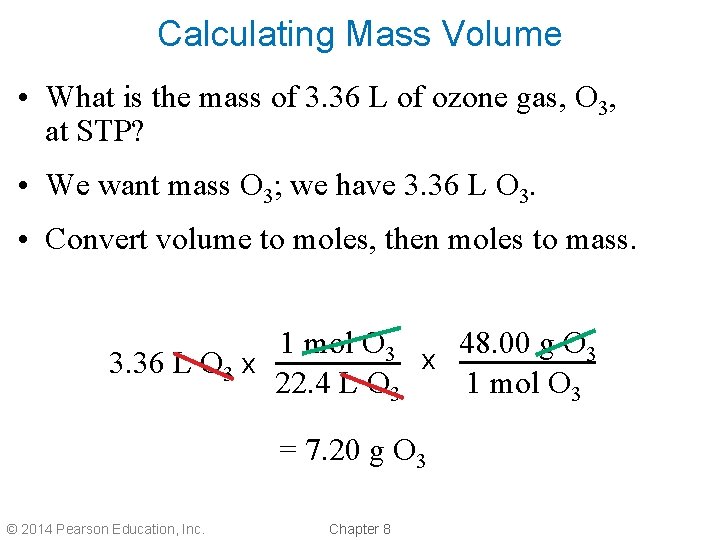
Calculating Mass Volume • What is the mass of 3. 36 L of ozone gas, O 3, at STP? • We want mass O 3; we have 3. 36 L O 3. • Convert volume to moles, then moles to mass. 1 mol O 3 48. 00 g O 3 x 3. 36 L O 3 x 22. 4 L O 3 1 mol O 3 = 7. 20 g O 3 © 2014 Pearson Education, Inc. Chapter 8

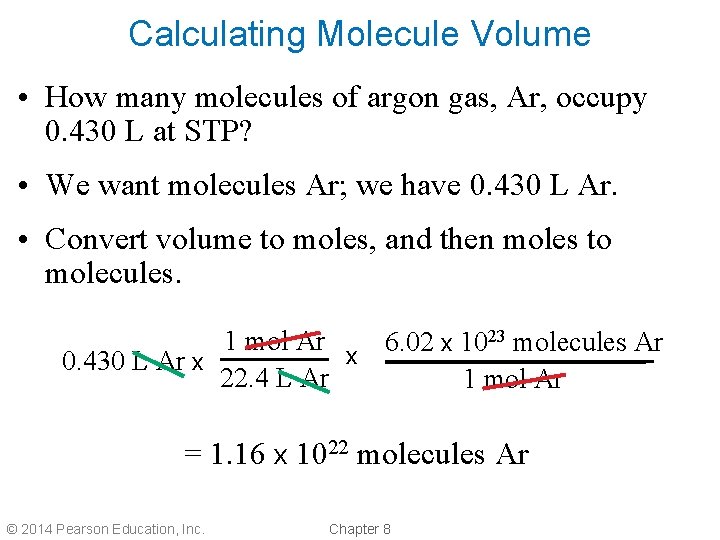
Calculating Molecule Volume • How many molecules of argon gas, Ar, occupy 0. 430 L at STP? • We want molecules Ar; we have 0. 430 L Ar. • Convert volume to moles, and then moles to molecules. 1 mol Ar 6. 02 x 1023 molecules Ar x 0. 430 L Ar x 22. 4 L Ar 1 mol Ar = 1. 16 x 1022 molecules Ar © 2014 Pearson Education, Inc. Chapter 8

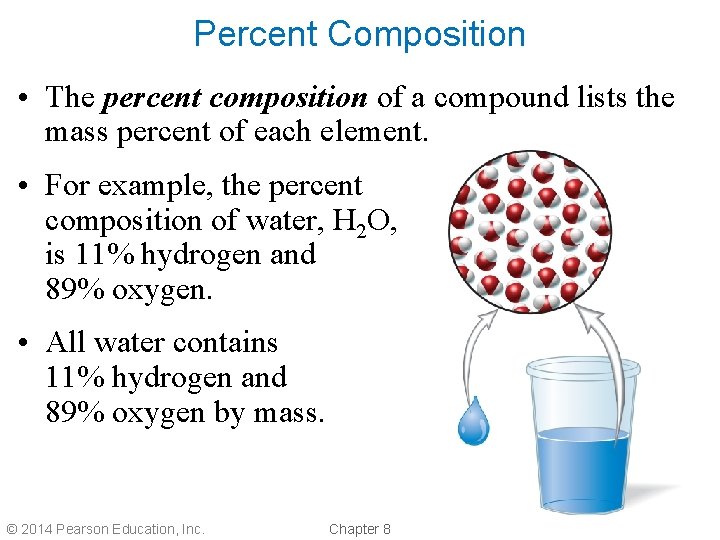
Percent Composition • The percent composition of a compound lists the mass percent of each element. • For example, the percent composition of water, H 2 O, is 11% hydrogen and 89% oxygen. • All water contains 11% hydrogen and 89% oxygen by mass. © 2014 Pearson Education, Inc. Chapter 8

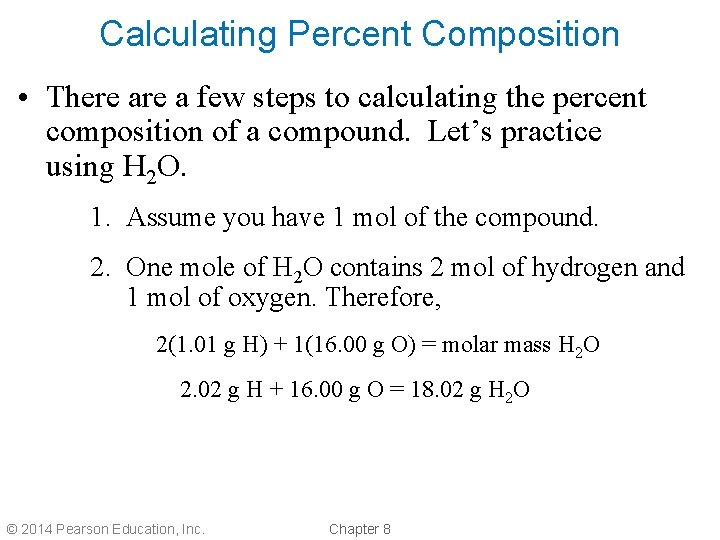
Calculating Percent Composition • There a few steps to calculating the percent composition of a compound. Let’s practice using H 2 O. 1. Assume you have 1 mol of the compound. 2. One mole of H 2 O contains 2 mol of hydrogen and 1 mol of oxygen. Therefore, 2(1. 01 g H) + 1(16. 00 g O) = molar mass H 2 O 2. 02 g H + 16. 00 g O = 18. 02 g H 2 O © 2014 Pearson Education, Inc. Chapter 8

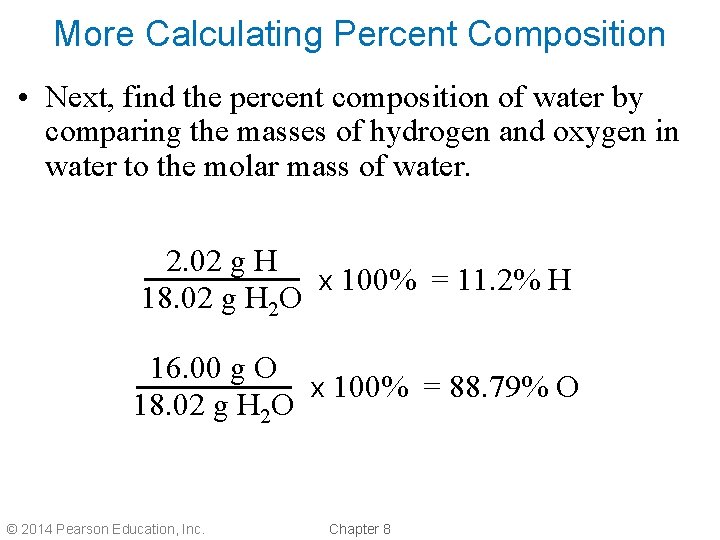
More Calculating Percent Composition • Next, find the percent composition of water by comparing the masses of hydrogen and oxygen in water to the molar mass of water. 2. 02 g H x 100% = 11. 2% H 18. 02 g H 2 O 16. 00 g O x 100% = 88. 79% O 18. 02 g H 2 O © 2014 Pearson Education, Inc. Chapter 8

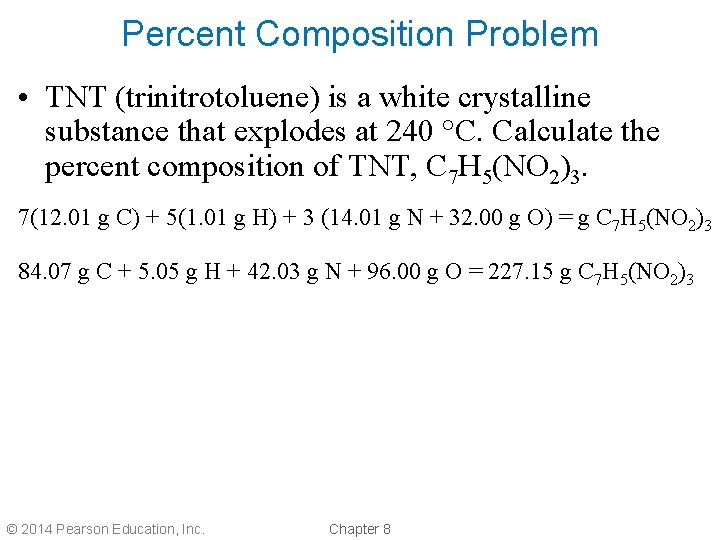
Percent Composition Problem • TNT (trinitrotoluene) is a white crystalline substance that explodes at 240 °C. Calculate the percent composition of TNT, C 7 H 5(NO 2)3. 7(12. 01 g C) + 5(1. 01 g H) + 3 (14. 01 g N + 32. 00 g O) = g C 7 H 5(NO 2)3 84. 07 g C + 5. 05 g H + 42. 03 g N + 96. 00 g O = 227. 15 g C 7 H 5(NO 2)3 © 2014 Pearson Education, Inc. Chapter 8

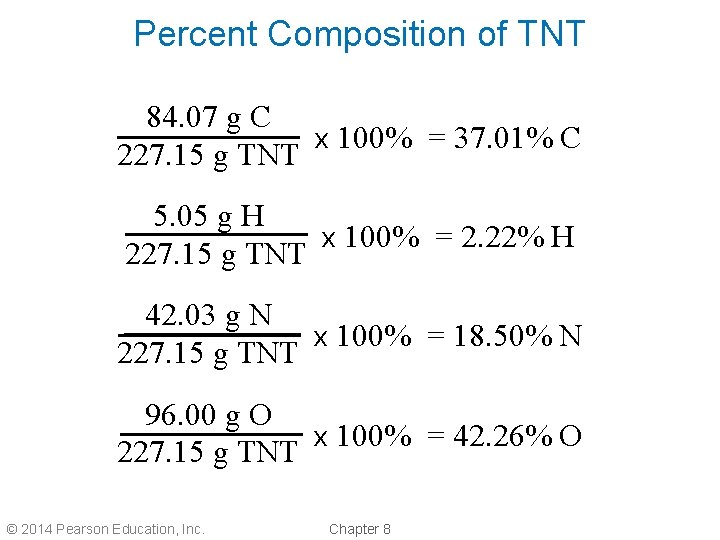
Percent Composition of TNT 84. 07 g C x 100% = 37. 01% C 227. 15 g TNT 5. 05 g H x 100% = 2. 22% H 227. 15 g TNT 42. 03 g N x 100% = 18. 50% N 227. 15 g TNT 96. 00 g O x 100% = 42. 26% O 227. 15 g TNT © 2014 Pearson Education, Inc. Chapter 8

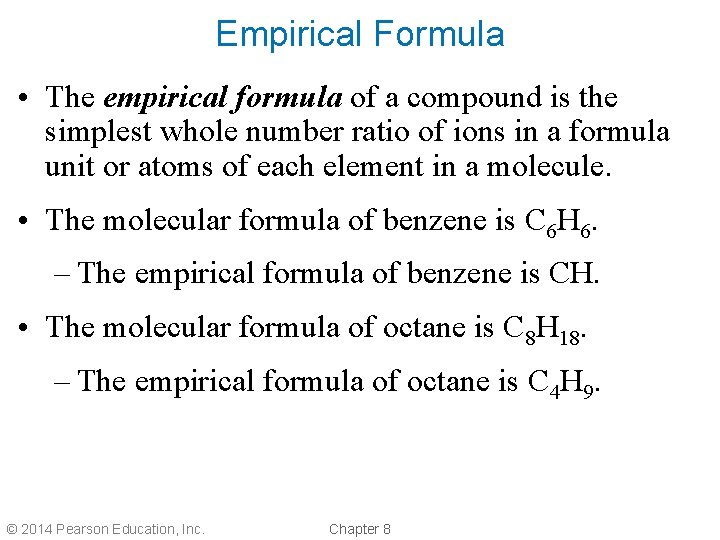
Empirical Formula • The empirical formula of a compound is the simplest whole number ratio of ions in a formula unit or atoms of each element in a molecule. • The molecular formula of benzene is C 6 H 6. – The empirical formula of benzene is CH. • The molecular formula of octane is C 8 H 18. – The empirical formula of octane is C 4 H 9. © 2014 Pearson Education, Inc. Chapter 8

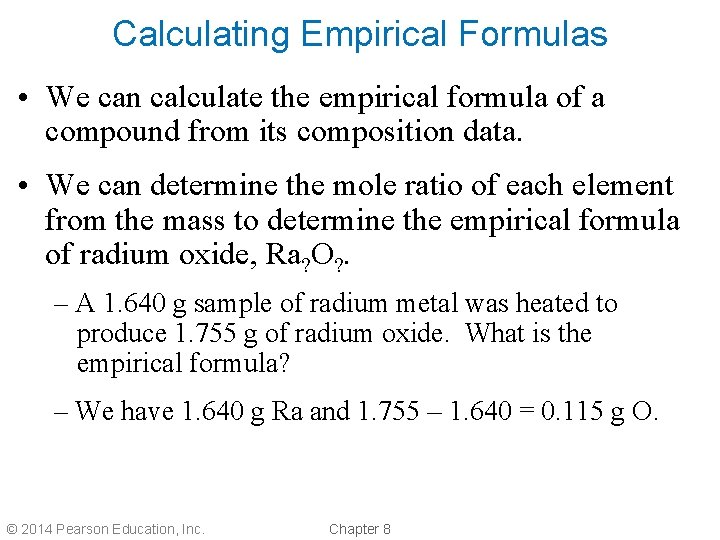
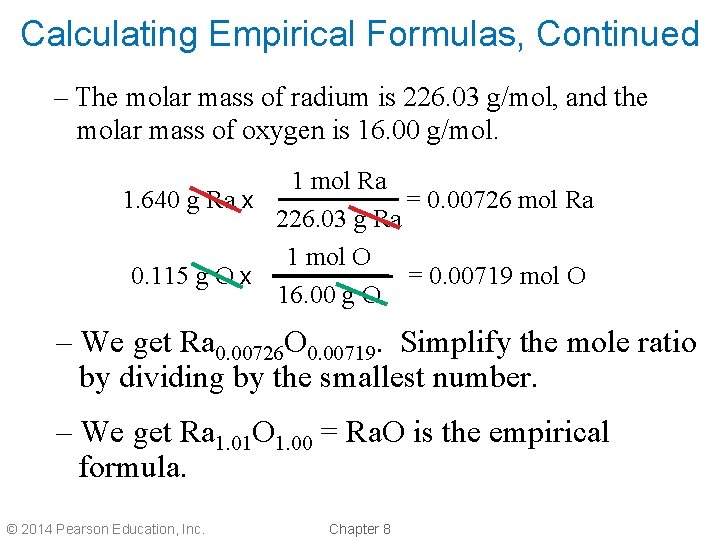
Calculating Empirical Formulas • We can calculate the empirical formula of a compound from its composition data. • We can determine the mole ratio of each element from the mass to determine the empirical formula of radium oxide, Ra? O? . – A 1. 640 g sample of radium metal was heated to produce 1. 755 g of radium oxide. What is the empirical formula? – We have 1. 640 g Ra and 1. 755 – 1. 640 = 0. 115 g O. © 2014 Pearson Education, Inc. Chapter 8

Calculating Empirical Formulas, Continued – The molar mass of radium is 226. 03 g/mol, and the molar mass of oxygen is 16. 00 g/mol. 1 mol Ra = 0. 00726 mol Ra 1. 640 g Ra x 226. 03 g Ra 1 mol O = 0. 00719 mol O 0. 115 g O x 16. 00 g O – We get Ra 0. 00726 O 0. 00719. Simplify the mole ratio by dividing by the smallest number. – We get Ra 1. 01 O 1. 00 = Ra. O is the empirical formula. © 2014 Pearson Education, Inc. Chapter 8

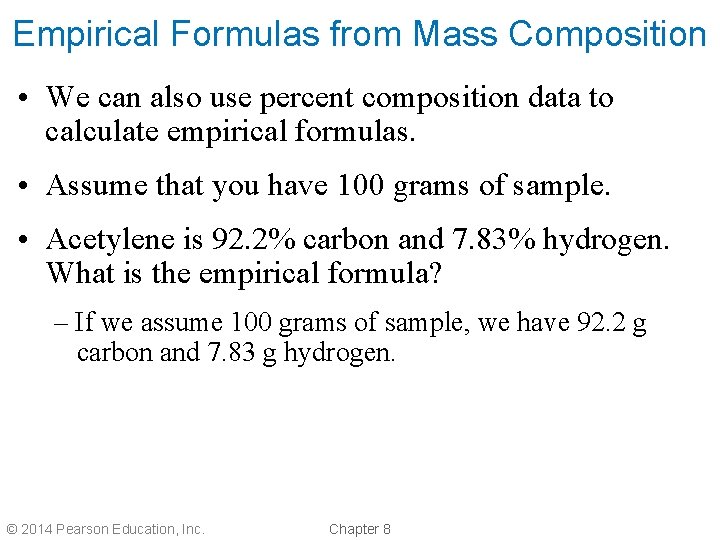
Empirical Formulas from Mass Composition • We can also use percent composition data to calculate empirical formulas. • Assume that you have 100 grams of sample. • Acetylene is 92. 2% carbon and 7. 83% hydrogen. What is the empirical formula? – If we assume 100 grams of sample, we have 92. 2 g carbon and 7. 83 g hydrogen. © 2014 Pearson Education, Inc. Chapter 8

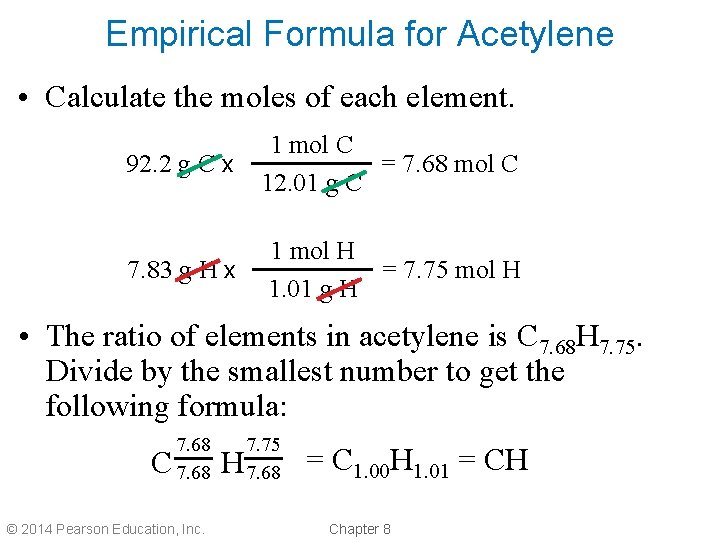
Empirical Formula for Acetylene • Calculate the moles of each element. 92. 2 g C x 1 mol C = 7. 68 mol C 12. 01 g C 7. 83 g H x 1 mol H = 7. 75 mol H 1. 01 g H • The ratio of elements in acetylene is C 7. 68 H 7. 75. Divide by the smallest number to get the following formula: C 7. 68 © 2014 Pearson Education, Inc. H 7. 75 7. 68 = C 1. 00 H 1. 01 = CH Chapter 8

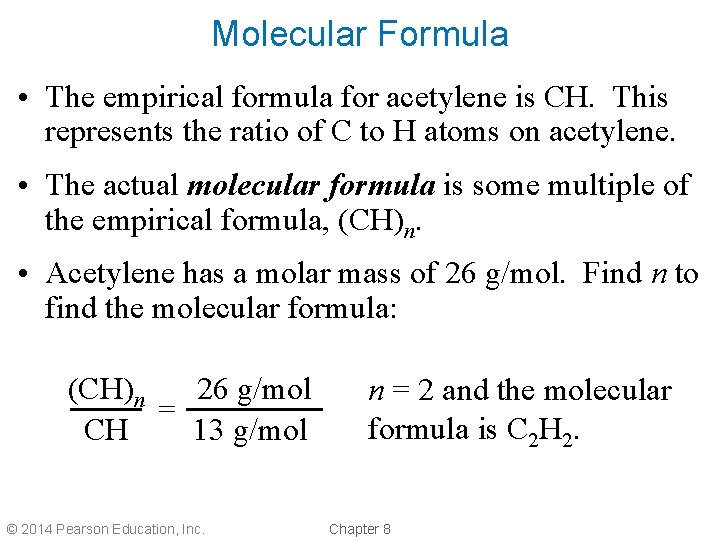
Molecular Formula • The empirical formula for acetylene is CH. This represents the ratio of C to H atoms on acetylene. • The actual molecular formula is some multiple of the empirical formula, (CH)n. • Acetylene has a molar mass of 26 g/mol. Find n to find the molecular formula: (CH)n 26 g/mol = 13 g/mol CH © 2014 Pearson Education, Inc. n = 2 and the molecular formula is C 2 H 2. Chapter 8

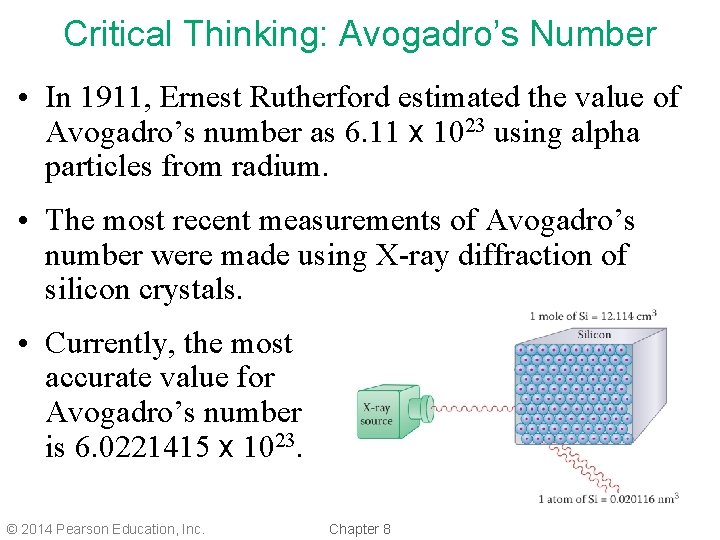
Critical Thinking: Avogadro’s Number • In 1911, Ernest Rutherford estimated the value of Avogadro’s number as 6. 11 x 1023 using alpha particles from radium. • The most recent measurements of Avogadro’s number were made using X-ray diffraction of silicon crystals. • Currently, the most accurate value for Avogadro’s number is 6. 0221415 x 1023. © 2014 Pearson Education, Inc. Chapter 8

Chapter Summary • Avogadro’s number is 6. 02 x 1023, and is 1 mole of any substance. • The molar mass of a substance is the sum of the atomic masses of each element in the formula. • At STP, 1 mole of any gas occupies 22. 4 L. © 2014 Pearson Education, Inc. Chapter 8

Chapter Summary, Continued • We can convert between the number of particles and moles of a substance using Avogadro’s number (1 mol = 6. 02 x 1023 particles). • We can convert between mass of a substance and moles of a substance using the molar mass. • We can convert between the volume of a gas at STP and moles of a gas using molar volume at STP (1 mol = 22. 4 L). © 2014 Pearson Education, Inc. Chapter 8

Chapter Summary, Continued • The percent composition of a substance is the mass percent of each element in that substance. • The empirical formula of a substance is the simplest whole number ratio of the elements in the formula. • The molecular formula is a multiple of the empirical formula. © 2014 Pearson Education, Inc. Chapter 8