Chapter 3 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical














- Slides: 50

Chapter 3 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Chapter 3 Matter and Energy by Christopher G. Hamaker Illinois State University © 2014 Pearson Education, Inc.

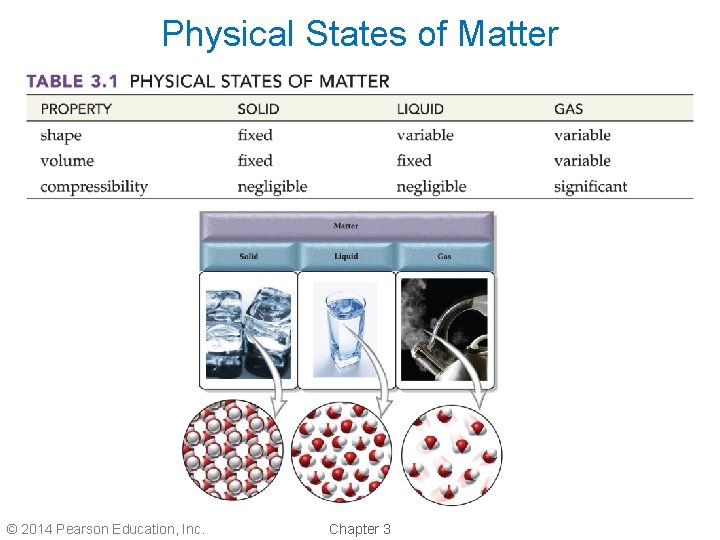
Matter • Matter is any substance that has mass and occupies volume. • Matter exists in one of three physical states: 1. Solid 2. Liquid 3. Gas © 2014 Pearson Education, Inc. Chapter 3

Solid State • In a solid, the particles of matter are tightly packed together. • Solids have a definite, fixed shape. • Solids cannot be compressed and have a definite volume. • Solids have the least energy of the three states of matter. © 2014 Pearson Education, Inc. Chapter 3

Liquid State • In a liquid, the particles of matter are loosely packed and are free to move past one another. • Liquids have an indefinite shape and assume the shape of their container. • Liquids cannot be compressed and have a definite volume. • Liquids have less energy than gases, but more energy than solids. © 2014 Pearson Education, Inc. Chapter 3

Gaseous State • In a gas, the particles of matter are far apart and uniformly distributed throughout the container. • Gases have an indefinite shape and assume the shape of their container. • Gases can be compressed and have an indefinite volume. • Gases have the most energy of the three states of matter. © 2014 Pearson Education, Inc. Chapter 3

Physical States of Matter © 2014 Pearson Education, Inc. Chapter 3

Changes in Physical States • Most substances can exist as either a solid, a liquid, or a gas. • Water exists as a solid below 0 °C; as a liquid between 0 °C and 100 °C; and as a gas above 100 °C. • A substance can change physical states as the temperature changes. © 2014 Pearson Education, Inc. Chapter 3

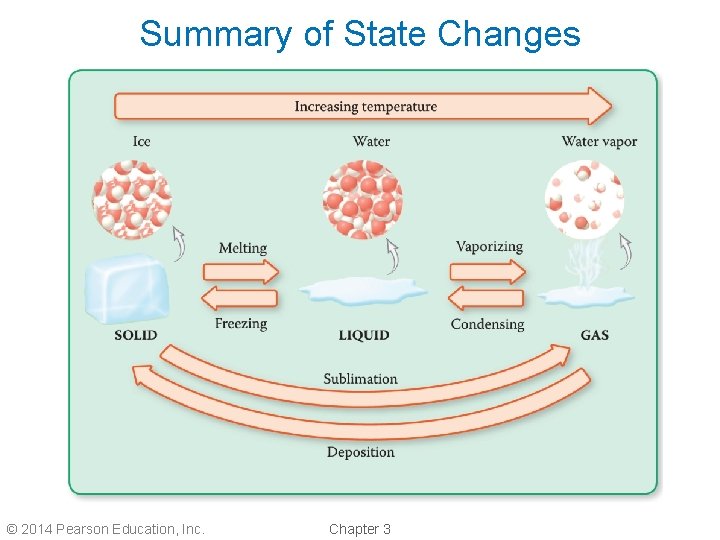
Solid ↔ Liquid Phase Changes • When a solid changes to a liquid, the phase change is called melting. • A substance melts as the temperature increases. • When a liquid changes to a solid, the phase change is called freezing. • A substance freezes as the temperature decreases. © 2014 Pearson Education, Inc. Chapter 3

Liquid ↔ Gas Phase Changes • When a liquid changes to a gas, the phase change is called vaporizing. • A substance vaporizes as the temperature increases. • When a gas changes to a liquid, the phase change is called condensing. • A substance condenses as the temperature decreases. © 2014 Pearson Education, Inc. Chapter 3

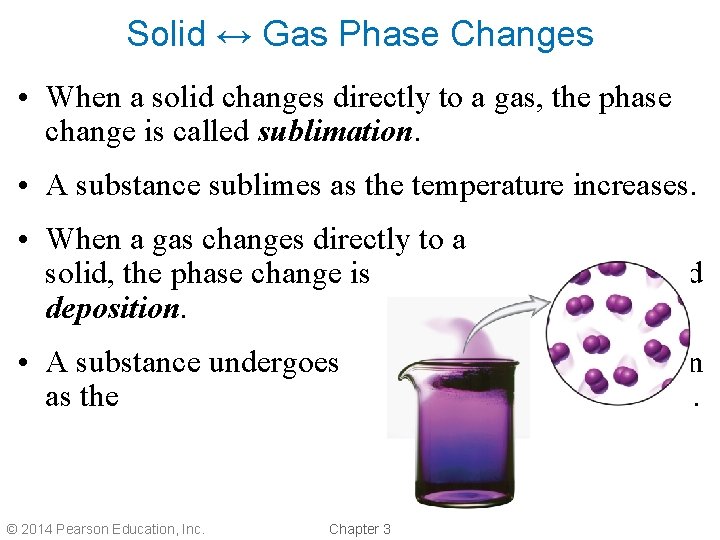
Solid ↔ Gas Phase Changes • When a solid changes directly to a gas, the phase change is called sublimation. • A substance sublimes as the temperature increases. • When a gas changes directly to a solid, the phase change is deposition. • A substance undergoes as the © 2014 Pearson Education, Inc. Chapter 3 called deposition temperature decreases.

Summary of State Changes © 2014 Pearson Education, Inc. Chapter 3

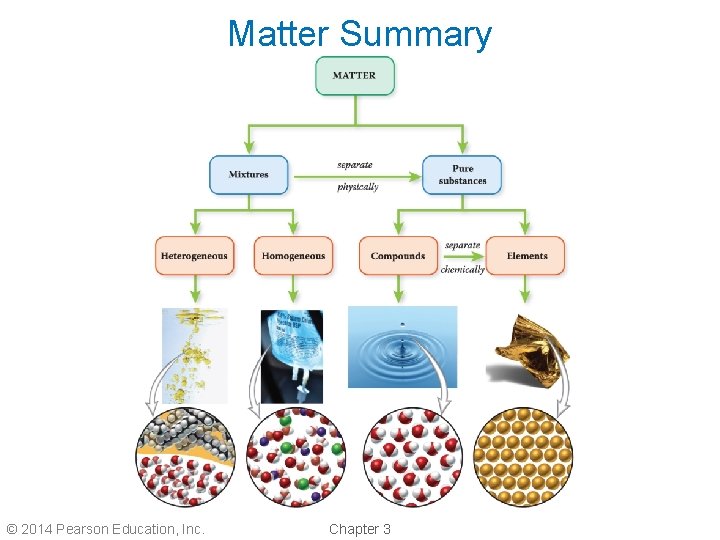
Classifications of Matter • Matter can be divided into two classes: 1. Mixtures 2. Pure substances • Mixtures are composed of more than one substance and can be physically separated into its component substances. • Pure substances are composed of only one substance and cannot be physically separated. © 2014 Pearson Education, Inc. Chapter 3

Mixtures • There are two types of mixtures: 1. Heterogeneous mixtures 2. Homogeneous mixtures • Heterogeneous mixtures do not have uniform properties throughout. – Sand water is a heterogeneous mixture. • Homogeneous mixtures have uniform properties throughout. – Salt water is a homogeneous mixture. © 2014 Pearson Education, Inc. Chapter 3

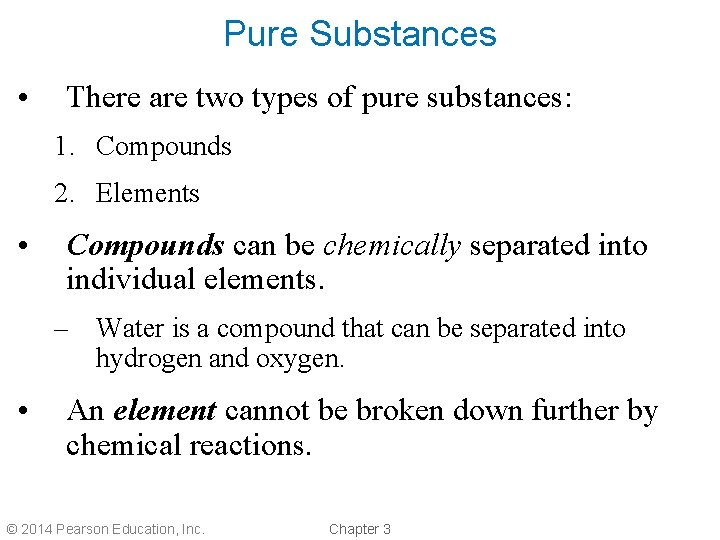
Pure Substances • There are two types of pure substances: 1. Compounds 2. Elements • Compounds can be chemically separated into individual elements. – Water is a compound that can be separated into hydrogen and oxygen. • An element cannot be broken down further by chemical reactions. © 2014 Pearson Education, Inc. Chapter 3

Matter Summary © 2014 Pearson Education, Inc. Chapter 3

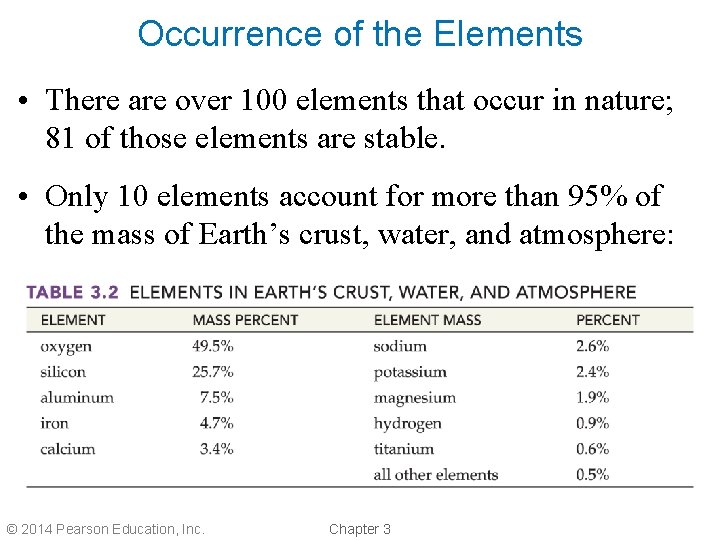
Occurrence of the Elements • There are over 100 elements that occur in nature; 81 of those elements are stable. • Only 10 elements account for more than 95% of the mass of Earth’s crust, water, and atmosphere: © 2014 Pearson Education, Inc. Chapter 3

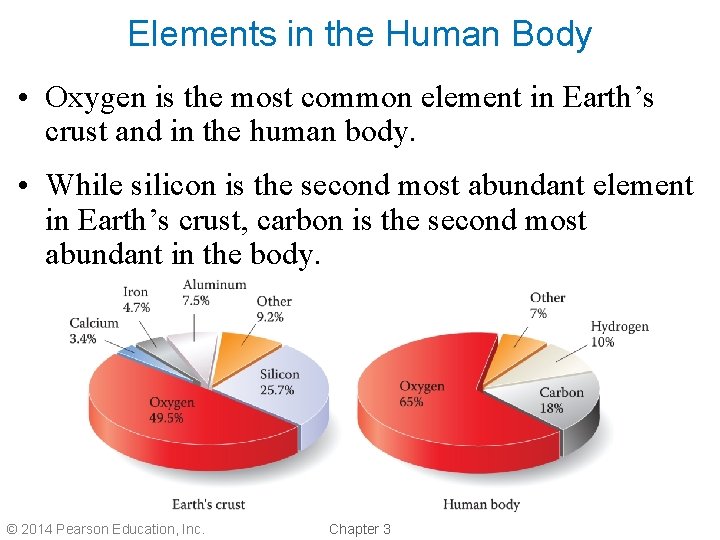
Elements in the Human Body • Oxygen is the most common element in Earth’s crust and in the human body. • While silicon is the second most abundant element in Earth’s crust, carbon is the second most abundant in the body. © 2014 Pearson Education, Inc. Chapter 3

Names of the Elements • Each element has a unique name. • Names have several origins: – Hydrogen is derived from Greek. – Carbon is derived from Latin. – Scandium is named for Scandinavia. – Curium is named for Marie Curie. – Nobelium is named for Alfred Nobel. © 2014 Pearson Education, Inc. Chapter 3

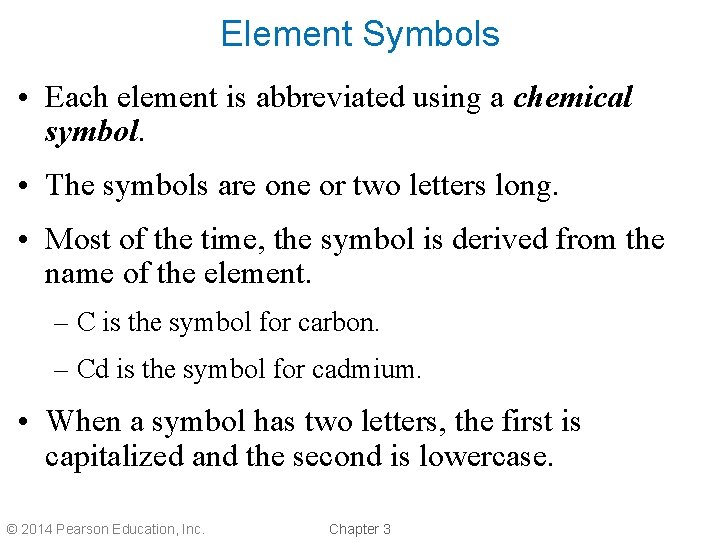
Element Symbols • Each element is abbreviated using a chemical symbol. • The symbols are one or two letters long. • Most of the time, the symbol is derived from the name of the element. – C is the symbol for carbon. – Cd is the symbol for cadmium. • When a symbol has two letters, the first is capitalized and the second is lowercase. © 2014 Pearson Education, Inc. Chapter 3

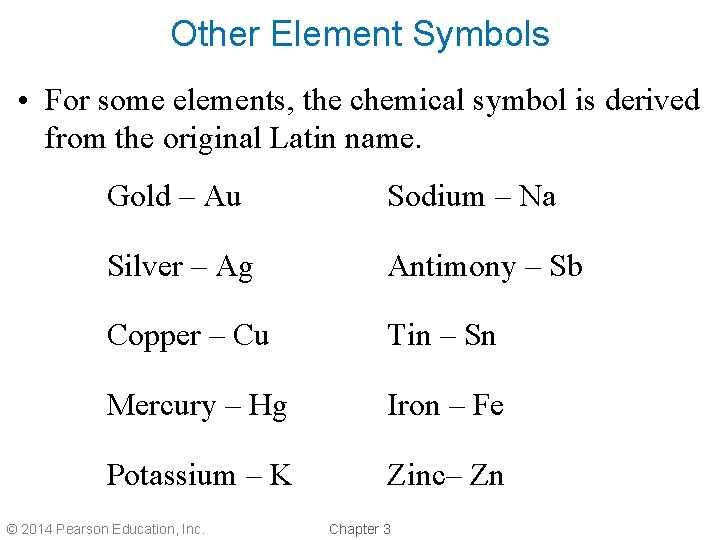
Other Element Symbols • For some elements, the chemical symbol is derived from the original Latin name. Gold – Au Sodium – Na Silver – Ag Antimony – Sb Copper – Cu Tin – Sn Mercury – Hg Iron – Fe Potassium – K Zinc– Zn © 2014 Pearson Education, Inc. Chapter 3

Critical Thinking: Aluminum or Aluminium? • Most metals have names that end in –ium. • However, element #13 is called aluminum in the USA and Canada, and aluminium in the rest of the world. • The different spelling is believed to be from a spelling error which caught on in the USA and Canada. • The official IUPAC name is “aluminium”; however, in 1993, IUPAC recognized the alternate spelling “aluminum. ” © 2014 Pearson Education, Inc. Chapter 3

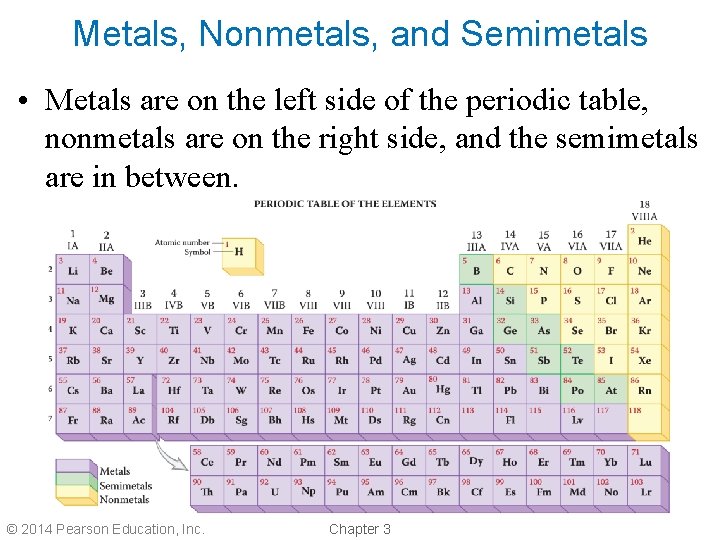
Types of Elements • Elements can be divided into three classes: 1. Metals 2. Nonmetals 3. Semimetals or metalloids • Semimetals have properties midway between those of metals and nonmetals. © 2014 Pearson Education, Inc. Chapter 3

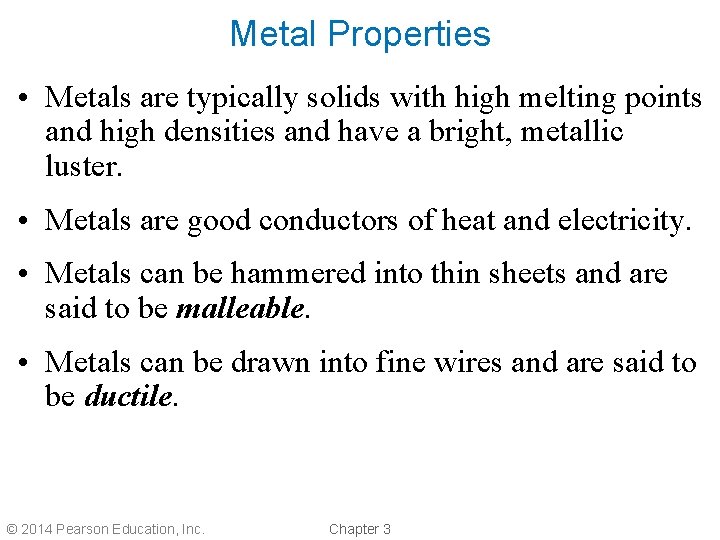
Metal Properties • Metals are typically solids with high melting points and high densities and have a bright, metallic luster. • Metals are good conductors of heat and electricity. • Metals can be hammered into thin sheets and are said to be malleable. • Metals can be drawn into fine wires and are said to be ductile. © 2014 Pearson Education, Inc. Chapter 3

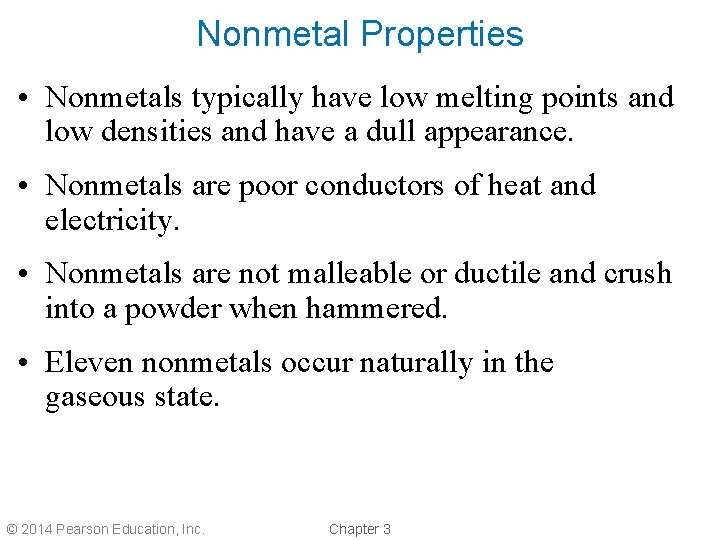
Nonmetal Properties • Nonmetals typically have low melting points and low densities and have a dull appearance. • Nonmetals are poor conductors of heat and electricity. • Nonmetals are not malleable or ductile and crush into a powder when hammered. • Eleven nonmetals occur naturally in the gaseous state. © 2014 Pearson Education, Inc. Chapter 3

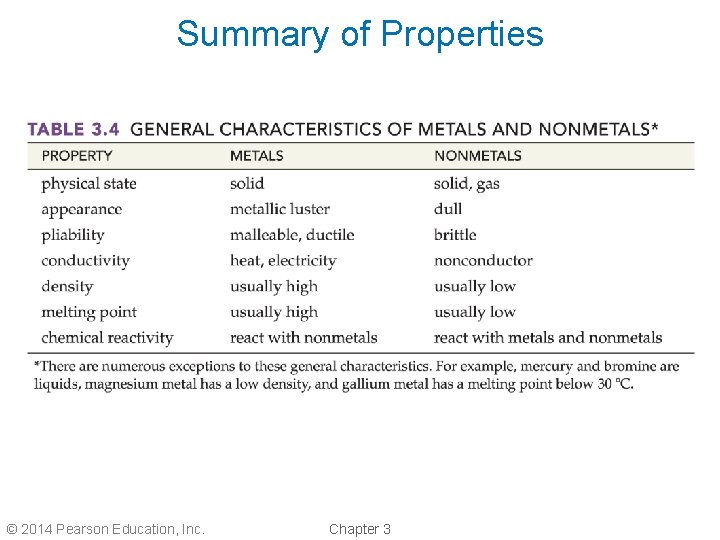
Summary of Properties © 2014 Pearson Education, Inc. Chapter 3

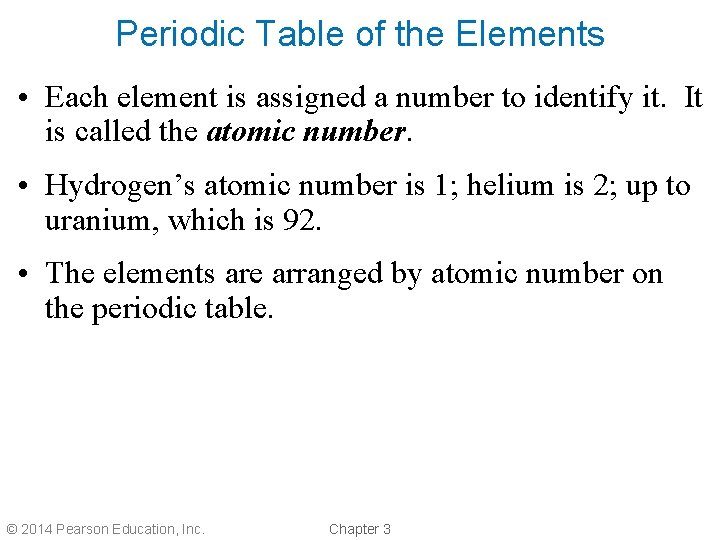
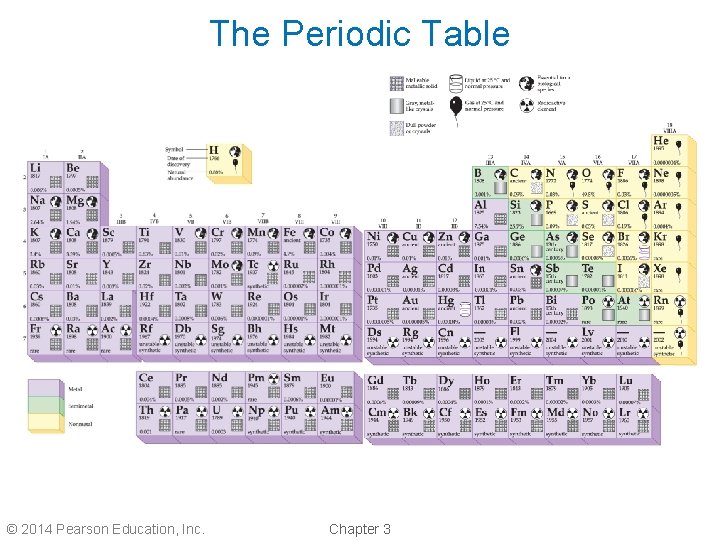
Periodic Table of the Elements • Each element is assigned a number to identify it. It is called the atomic number. • Hydrogen’s atomic number is 1; helium is 2; up to uranium, which is 92. • The elements are arranged by atomic number on the periodic table. © 2014 Pearson Education, Inc. Chapter 3

The Periodic Table © 2014 Pearson Education, Inc. Chapter 3

Metals, Nonmetals, and Semimetals • Metals are on the left side of the periodic table, nonmetals are on the right side, and the semimetals are in between. © 2014 Pearson Education, Inc. Chapter 3

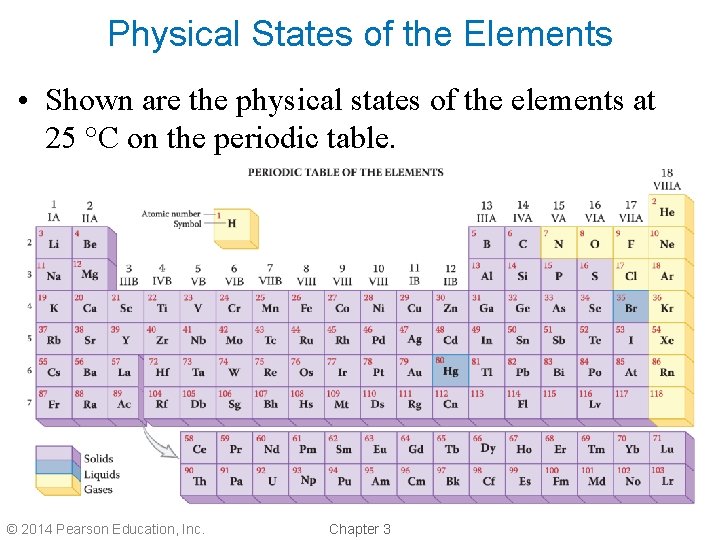
Physical States of the Elements • Shown are the physical states of the elements at 25 °C on the periodic table. © 2014 Pearson Education, Inc. Chapter 3

Chemistry Connection: Elements 104 and Beyond • Scientists continue to discover new, heavier elements beyond the current periodic table. • Sometimes disagreements arise over naming of the new elements. • IUPAC assigns names to new elements. • Until IUPAC assigns a name, the elements are named using Latin prefixes for the numbers followed by the suffix –ium. – Hence, element 104 is unnilquadium. © 2014 Pearson Education, Inc. Chapter 3

Law of Definite Composition • The law of definite composition states that “Compounds always contain the same elements in a constant proportion by mass. ” • Water is always 11. 2% hydrogen and 88. 8% oxygen by mass, no matter what its source. © 2014 Pearson Education, Inc. Chapter 3

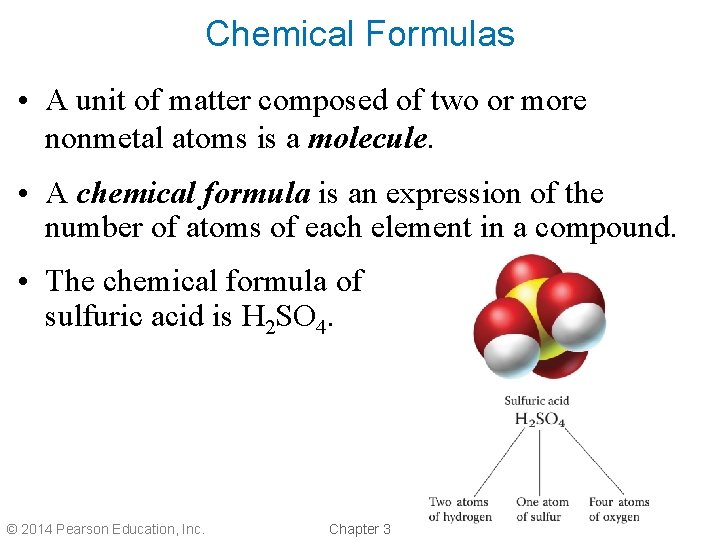
Chemical Formulas • A unit of matter composed of two or more nonmetal atoms is a molecule. • A chemical formula is an expression of the number of atoms of each element in a compound. • The chemical formula of sulfuric acid is H 2 SO 4. © 2014 Pearson Education, Inc. Chapter 3

Writing Chemical Formulas • The number of each type of atom in a molecule is indicated with a subscript in a chemical formula. • If there is only one atom of a certain type, no “ 1” is used. • A molecule of vitamin B 3 has 6 carbon atoms, 6 hydrogen atoms, 2 nitrogen atoms, and 1 oxygen atom. What is the chemical formula? C 6 H 6 N 2 O © 2014 Pearson Education, Inc. Chapter 3

Interpreting Chemical Formulas • Some chemical formulas use parentheses to clarify atomic composition. • Ethylene glycol, a component of some antifreezes, has a chemical formula of C 2 H 4(OH)2. It contains 2 carbon atoms, 4 hydrogen atoms, and 2 OH units, for a total of 6 hydrogen atoms and 2 oxygen atoms. How many total atoms are in ethylene glycol? • Ethylene glycol has a total of 10 atoms. © 2014 Pearson Education, Inc. Chapter 3

Physical and Chemical Properties • A physical property is a characteristic of a pure substance that we can observe without changing its composition. • Physical properties include appearance, melting and boiling points, density, heat and electrical conductivity, solubility, and physical state. • A chemical property of a pure substance describes its chemical reactions with other substances. © 2014 Pearson Education, Inc. Chapter 3

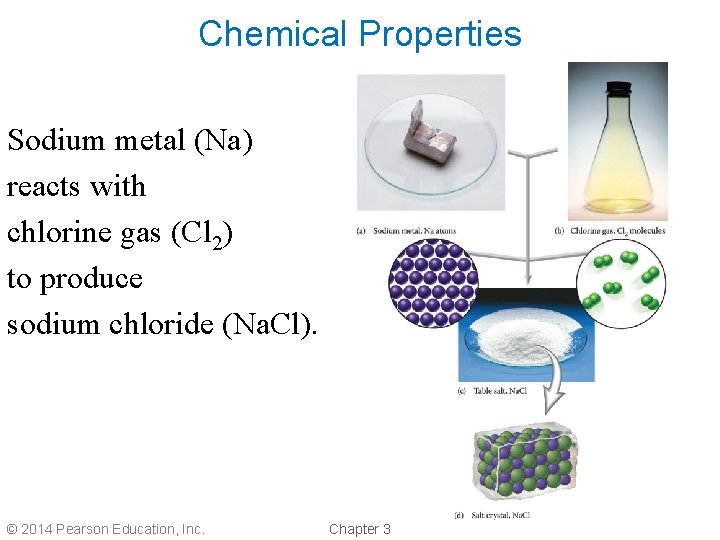
Chemical Properties Sodium metal (Na) reacts with chlorine gas (Cl 2) to produce sodium chloride (Na. Cl). © 2014 Pearson Education, Inc. Chapter 3

Physical and Chemical Change • A physical change is a change where the chemical composition of the sample does not change. • These include changes in physical state or shape of a pure substance. • A chemical change is a chemical reaction. • The composition of the sample changes during a chemical change. © 2014 Pearson Education, Inc. Chapter 3

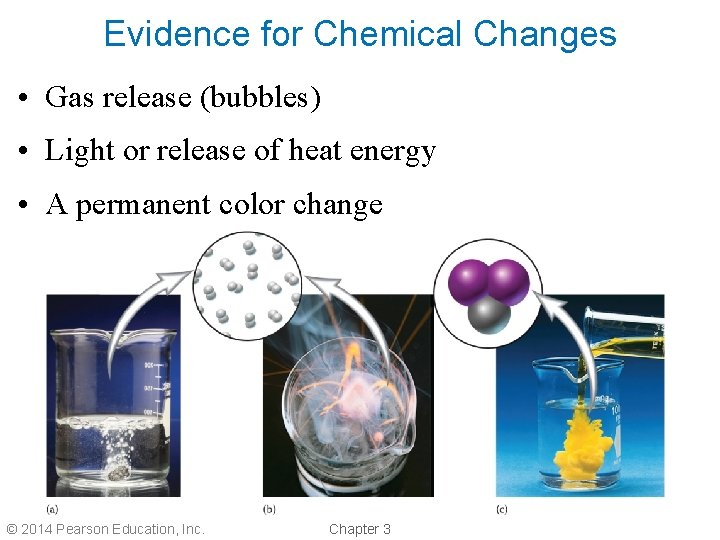
Evidence for Chemical Changes • Gas release (bubbles) • Light or release of heat energy • A permanent color change © 2014 Pearson Education, Inc. Chapter 3

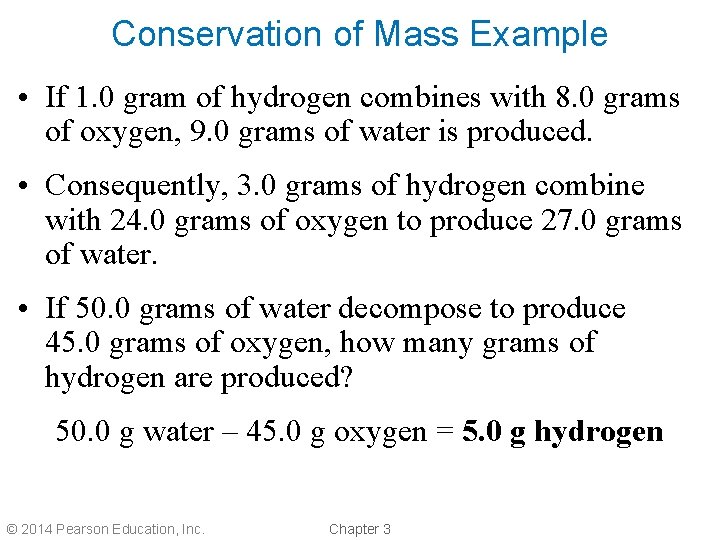
Conservation of Mass • Antoine Lavoisier found that the mass of reactants before a chemical change was always equal to the mass of products after a chemical change. • This is the law of conservation of mass. • Matter is neither created nor destroyed in a chemical reaction. © 2014 Pearson Education, Inc. Chapter 3

Conservation of Mass Example • If 1. 0 gram of hydrogen combines with 8. 0 grams of oxygen, 9. 0 grams of water is produced. • Consequently, 3. 0 grams of hydrogen combine with 24. 0 grams of oxygen to produce 27. 0 grams of water. • If 50. 0 grams of water decompose to produce 45. 0 grams of oxygen, how many grams of hydrogen are produced? 50. 0 g water – 45. 0 g oxygen = 5. 0 g hydrogen © 2014 Pearson Education, Inc. Chapter 3

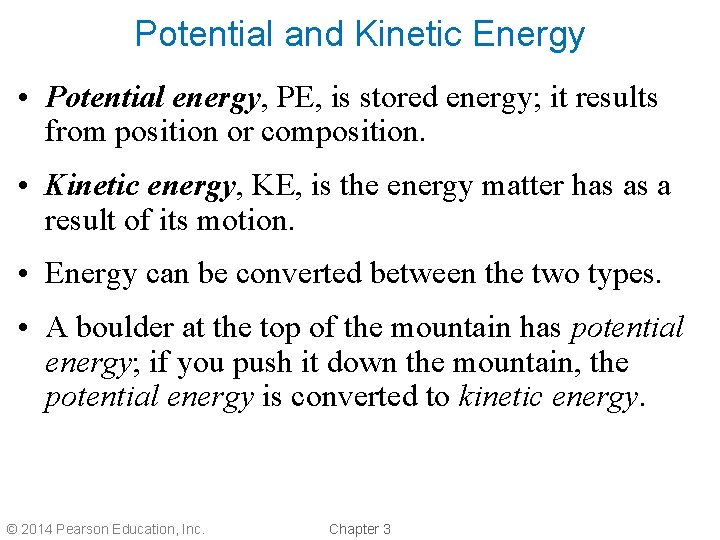
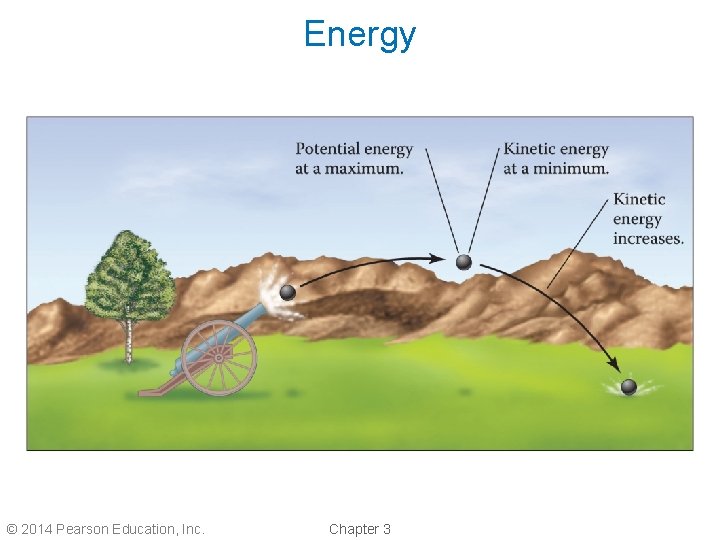
Potential and Kinetic Energy • Potential energy, PE, is stored energy; it results from position or composition. • Kinetic energy, KE, is the energy matter has as a result of its motion. • Energy can be converted between the two types. • A boulder at the top of the mountain has potential energy; if you push it down the mountain, the potential energy is converted to kinetic energy. © 2014 Pearson Education, Inc. Chapter 3

Energy © 2014 Pearson Education, Inc. Chapter 3

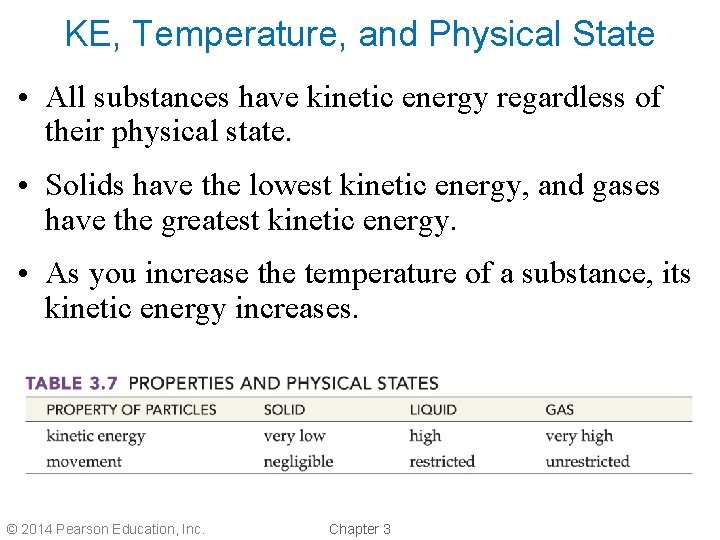
KE, Temperature, and Physical State • All substances have kinetic energy regardless of their physical state. • Solids have the lowest kinetic energy, and gases have the greatest kinetic energy. • As you increase the temperature of a substance, its kinetic energy increases. © 2014 Pearson Education, Inc. Chapter 3

Law of Conservation of Energy • Just like matter, energy cannot be created or destroyed, but it can be converted from one form to another. • This is the law of conservation of energy. • There are six forms of energy: 1. 2. 3. 4. 5. 6. Heat Light Chemical Electrical Mechanical Nuclear © 2014 Pearson Education, Inc. Chapter 3

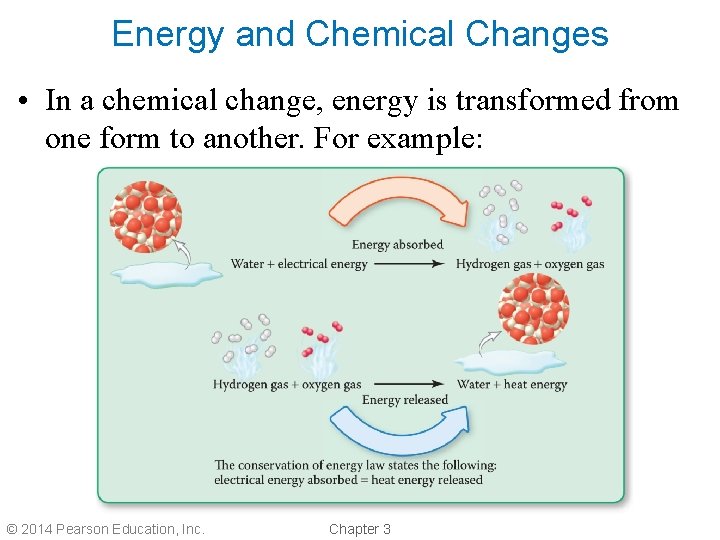
Energy and Chemical Changes • In a chemical change, energy is transformed from one form to another. For example: © 2014 Pearson Education, Inc. Chapter 3

Critical Thinking: Lower Gasoline Bills • In terms of expense, is it better to fill a gas tank in the cool morning, or in the warm afternoon? • No matter the temperature, the number of gallons delivered is always the same. • When the temperature is lower, a greater mass of gasoline is delivered for the same volume. • However, the difference in mass between 40°F and 70°F is only about 1%. © 2014 Pearson Education, Inc. Chapter 3

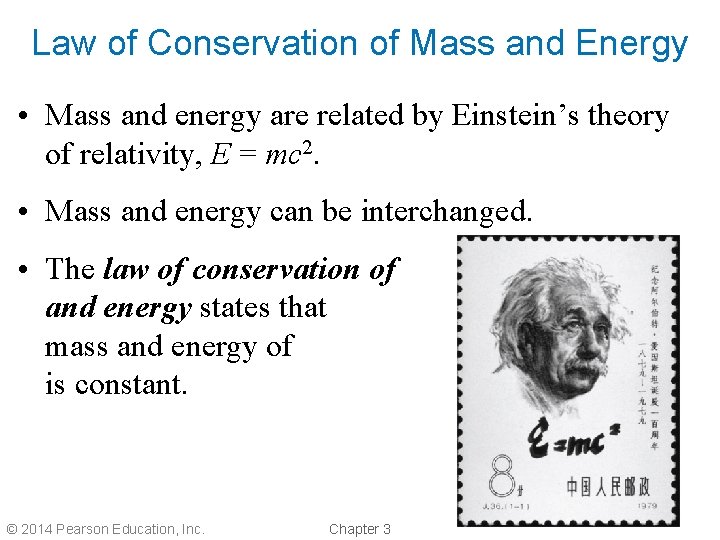
Law of Conservation of Mass and Energy • Mass and energy are related by Einstein’s theory of relativity, E = mc 2. • Mass and energy can be interchanged. • The law of conservation of and energy states that mass and energy of is constant. © 2014 Pearson Education, Inc. Chapter 3 mass the total the universe

Chapter Summary • Matter exists in three physical states: 1. Solid 2. Liquid 3. Gas • Substances can be converted between the three states. • Substances can be mixtures or pure substances. © 2014 Pearson Education, Inc. Chapter 3

Chapter Summary, Continued • Pure substances can be either compounds or elements. • The elements are arranged in the periodic table. • Each element has a name and a one- or two-letter symbol. • Elements are classified as either metals, nonmetals, or semimetals. © 2014 Pearson Education, Inc. Chapter 3

Chapter Summary, Continued • A physical change is a change in physical state or shape. • A chemical change is a change in the chemical composition of a substance. • Both mass and energy are conserved in chemical and physical changes. © 2014 Pearson Education, Inc. Chapter 3