Chapter 13 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical













- Slides: 38

Chapter 13 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Chapter 13 Solutions by Christopher G. Hamaker Illinois State University © 2014 Pearson Education, Inc.

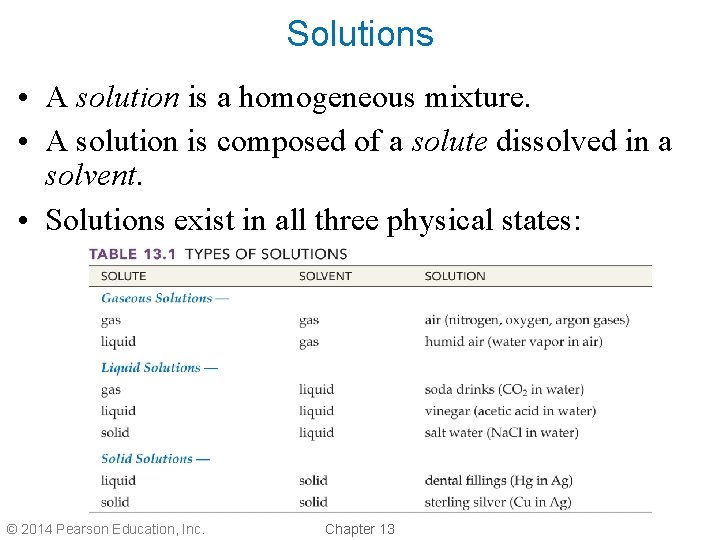
Solutions • A solution is a homogeneous mixture. • A solution is composed of a solute dissolved in a solvent. • Solutions exist in all three physical states: © 2014 Pearson Education, Inc. Chapter 13

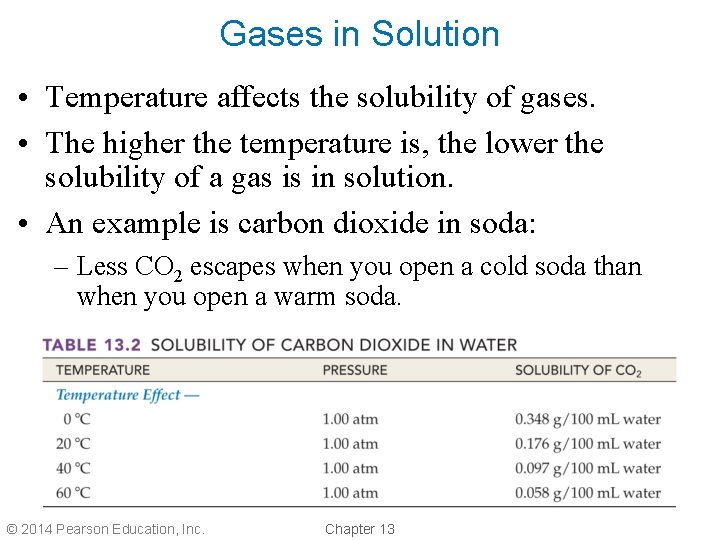
Gases in Solution • Temperature affects the solubility of gases. • The higher the temperature is, the lower the solubility of a gas is in solution. • An example is carbon dioxide in soda: – Less CO 2 escapes when you open a cold soda than when you open a warm soda. © 2014 Pearson Education, Inc. Chapter 13

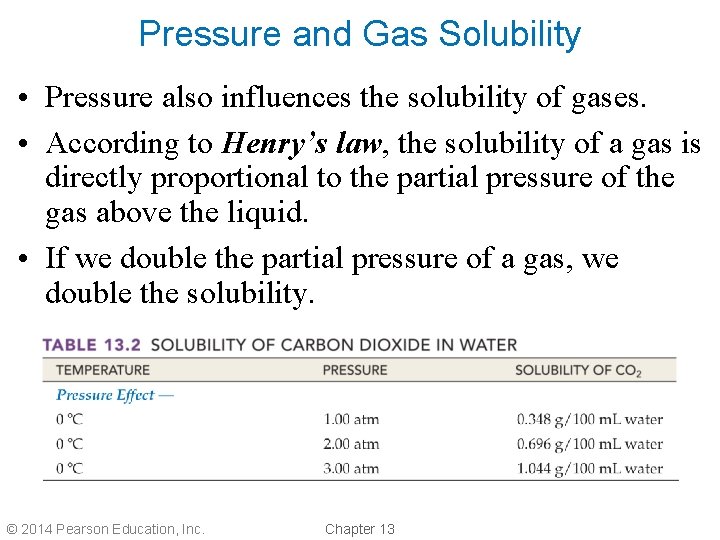
Pressure and Gas Solubility • Pressure also influences the solubility of gases. • According to Henry’s law, the solubility of a gas is directly proportional to the partial pressure of the gas above the liquid. • If we double the partial pressure of a gas, we double the solubility. © 2014 Pearson Education, Inc. Chapter 13

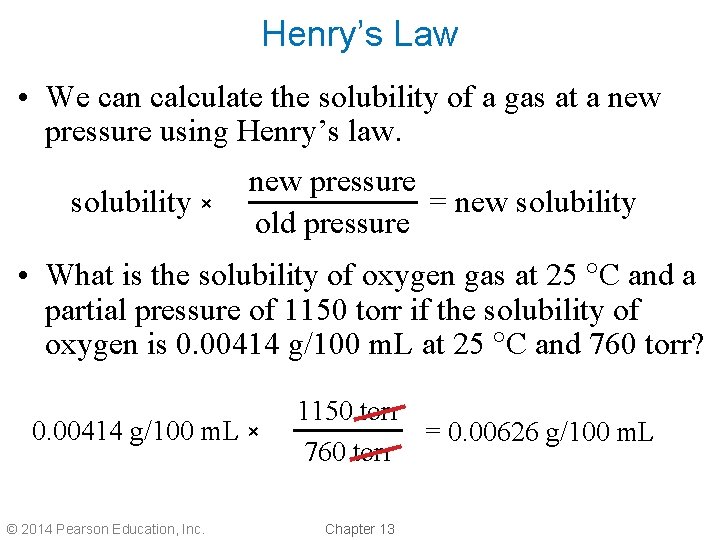
Henry’s Law • We can calculate the solubility of a gas at a new pressure using Henry’s law. solubility × new pressure = new solubility old pressure • What is the solubility of oxygen gas at 25 C and a partial pressure of 1150 torr if the solubility of oxygen is 0. 00414 g/100 m. L at 25 C and 760 torr? 0. 00414 g/100 m. L × © 2014 Pearson Education, Inc. 1150 torr = 0. 00626 g/100 m. L 760 torr Chapter 13

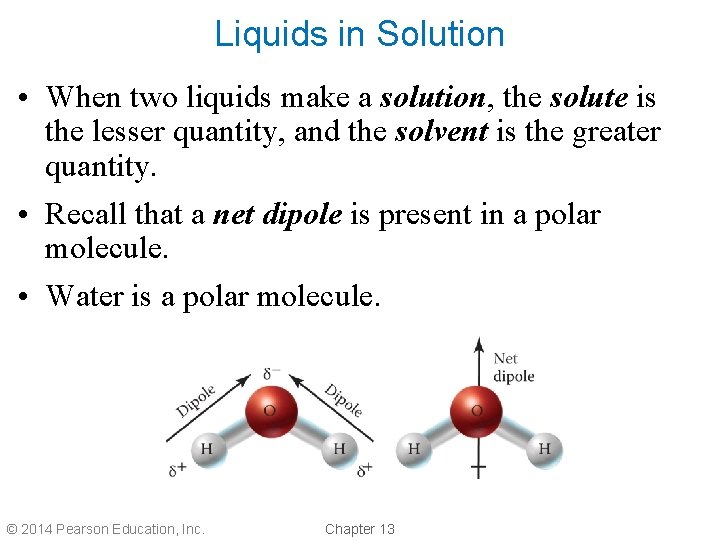
Liquids in Solution • When two liquids make a solution, the solute is the lesser quantity, and the solvent is the greater quantity. • Recall that a net dipole is present in a polar molecule. • Water is a polar molecule. © 2014 Pearson Education, Inc. Chapter 13

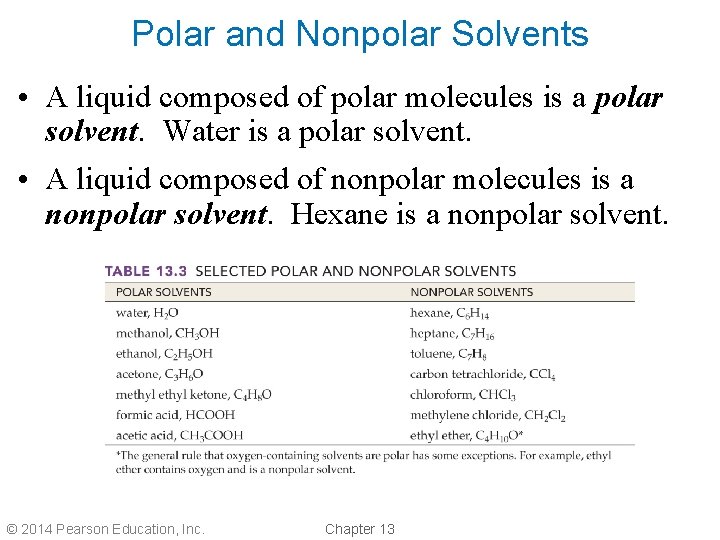
Polar and Nonpolar Solvents • A liquid composed of polar molecules is a polar solvent. Water is a polar solvent. • A liquid composed of nonpolar molecules is a nonpolar solvent. Hexane is a nonpolar solvent. © 2014 Pearson Education, Inc. Chapter 13

Like Dissolves Like Rule • • Polar solvents dissolve in one another. Nonpolar solvents dissolve in one another. This is the like dissolves like rule. Methanol dissolves in water, but hexane does not dissolve in water. • Hexane dissolves in toluene, but water does not dissolve in toluene. © 2014 Pearson Education, Inc. Chapter 13

Miscible and Immiscible • Two liquids that completely dissolve in each other are miscible liquids. • Two liquids that are not miscible in each other are immiscible liquids. • Polar water and nonpolar oil are immiscible liquids and do not mix to form a solution. © 2014 Pearson Education, Inc. Chapter 13

Solids in Solution • When a solid substance dissolves in a liquid, the solute particles are attracted to the solvent particles. • When a solution forms, the solute particles are more strongly attracted to the solvent particles than to each other. • We can also predict whether a solid will dissolve in a liquid by applying the like dissolves like rule. © 2014 Pearson Education, Inc. Chapter 13

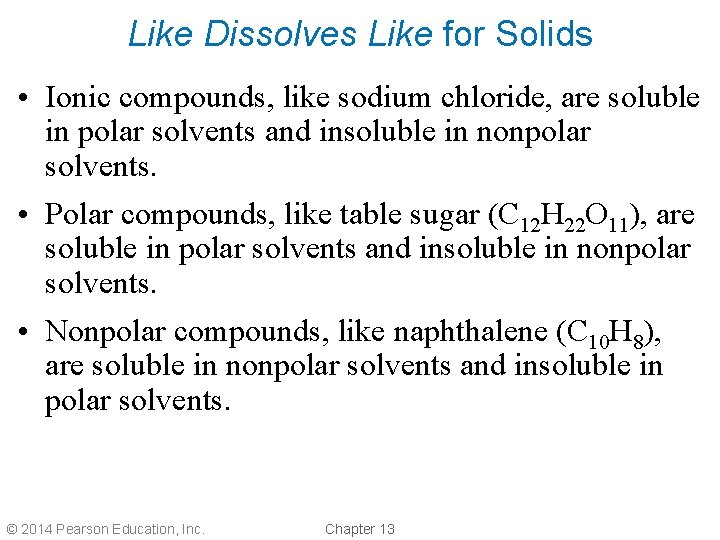
Like Dissolves Like for Solids • Ionic compounds, like sodium chloride, are soluble in polar solvents and insoluble in nonpolar solvents. • Polar compounds, like table sugar (C 12 H 22 O 11), are soluble in polar solvents and insoluble in nonpolar solvents. • Nonpolar compounds, like naphthalene (C 10 H 8), are soluble in nonpolar solvents and insoluble in polar solvents. © 2014 Pearson Education, Inc. Chapter 13

Chemistry Connection: Colloids • Why is the beam of light not visible in the solution, but observed in the colloid? • The solution, at the right of this slide, is a colloid. • A colloid is a solution with large particles (ranging from 1 to 100 nm). • The solute particles in a colloid are large enough to scatter light via a phenomenon known as the Tyndall effect. © 2014 Pearson Education, Inc. Chapter 13

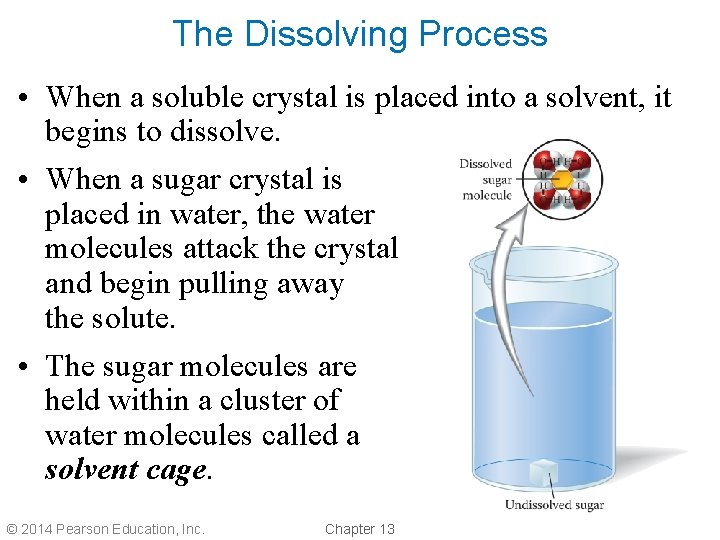
The Dissolving Process • When a soluble crystal is placed into a solvent, it begins to dissolve. • When a sugar crystal is placed in water, the water molecules attack the crystal and begin pulling away the solute. • The sugar molecules are held within a cluster of water molecules called a solvent cage. © 2014 Pearson Education, Inc. Chapter 13

Dissolving of Ionic Compounds • When a sodium chloride crystal is placed in water, the water molecules attack the edge of the crystal. • In an ionic compound, the water molecules pull away positive and negative ions. • The anions are surrounded by the positively charged hydrogen atoms on water. • The cations are surrounded by the negatively charged oxygen on water. © 2014 Pearson Education, Inc. Chapter 13

Rate of Dissolving • There are three ways we can speed up the rate of dissolving for a solid compound: 1. Heating the solution • This increases the kinetic energy of the solvent, and the solute is attacked faster by the solvent molecules. 2. Stirring the solution • This increases the interaction between water molecules and solute. 3. Grinding the solute • There is more surface area for the solvent to attack. © 2014 Pearson Education, Inc. Chapter 13

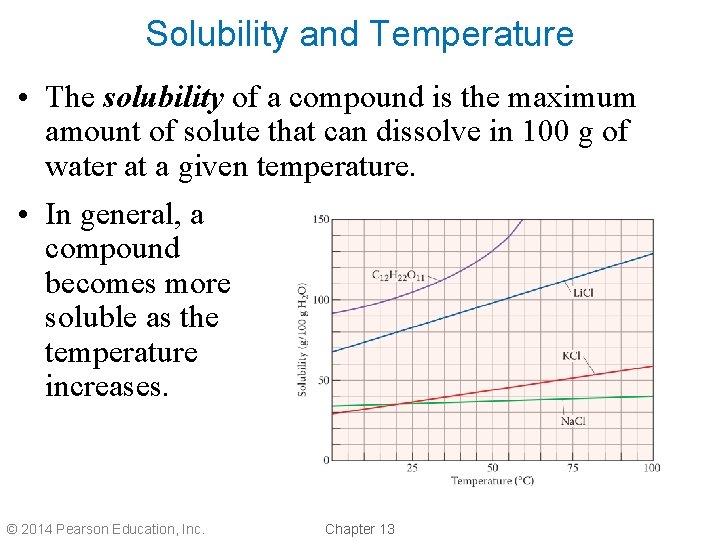
Solubility and Temperature • The solubility of a compound is the maximum amount of solute that can dissolve in 100 g of water at a given temperature. • In general, a compound becomes more soluble as the temperature increases. © 2014 Pearson Education, Inc. Chapter 13

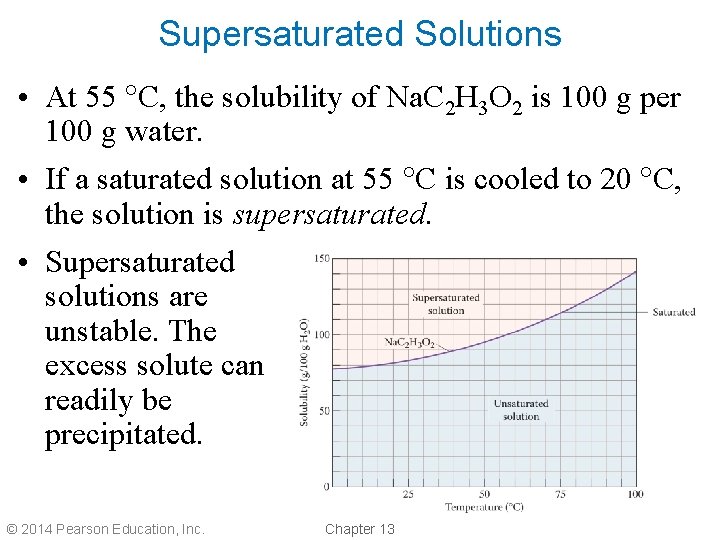
Saturated Solutions • A solution containing exactly the maximum amount of solute at a given temperature is a saturated solution. • A solution that contains less than the maximum amount of solute is an unsaturated solution. • Under certain conditions, it is possible to exceed the maximum solubility of a compound. A solution with greater than the maximum amount of solute is a supersaturated solution. © 2014 Pearson Education, Inc. Chapter 13

Supersaturated Solutions • At 55 C, the solubility of Na. C 2 H 3 O 2 is 100 g per 100 g water. • If a saturated solution at 55 C is cooled to 20 C, the solution is supersaturated. • Supersaturated solutions are unstable. The excess solute can readily be precipitated. © 2014 Pearson Education, Inc. Chapter 13

Supersaturation • A single crystal of sodium acetate added to a supersaturated solution of sodium acetate in water causes the excess solute to rapidly crystallize from the solution. © 2014 Pearson Education, Inc. Chapter 13

Mass/Mass Percent Concentration • The concentration of a solution tells us how much solute is dissolved in a given quantity of solution. • We often hear imprecise terms such as a “dilute solution” or a “concentrated solution. ” • There are two precise ways to express the concentration of a solution: 1. Mass/mass percent 2. Molarity © 2014 Pearson Education, Inc. Chapter 13

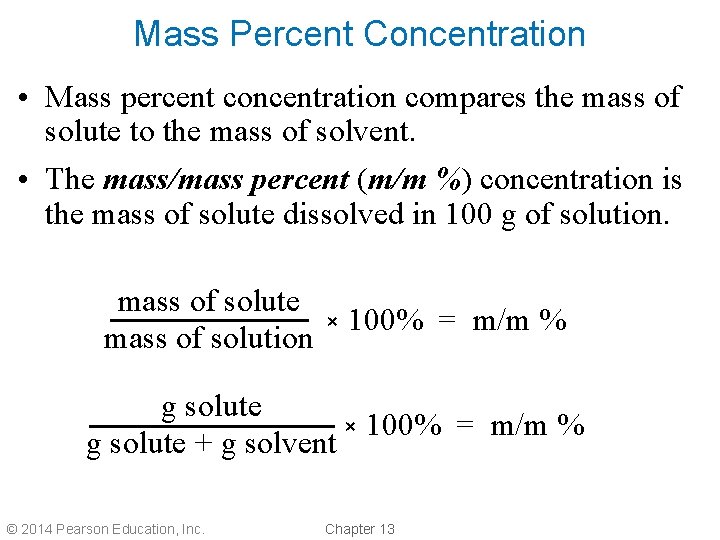
Mass Percent Concentration • Mass percent concentration compares the mass of solute to the mass of solvent. • The mass/mass percent (m/m %) concentration is the mass of solute dissolved in 100 g of solution. mass of solute mass of solution × 100% = m/m % g solute + g solvent © 2014 Pearson Education, Inc. Chapter 13

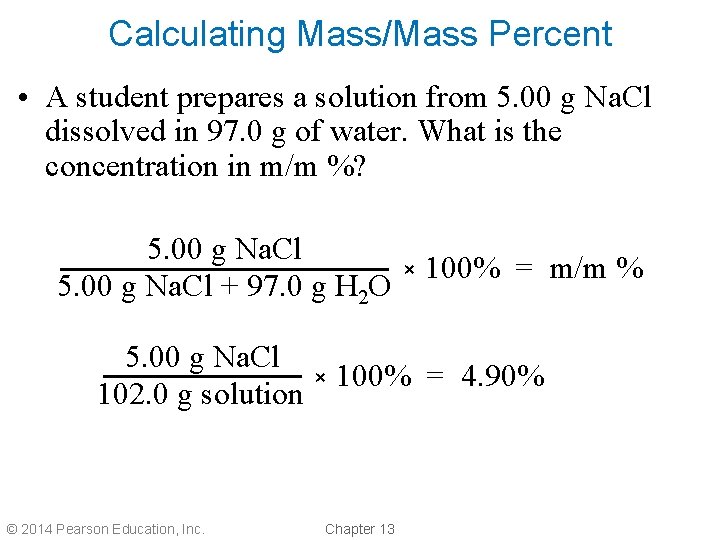
Calculating Mass/Mass Percent • A student prepares a solution from 5. 00 g Na. Cl dissolved in 97. 0 g of water. What is the concentration in m/m %? 5. 00 g Na. Cl + 97. 0 g H 2 O × 100% = m/m % 5. 00 g Na. Cl × 100% = 4. 90% 102. 0 g solution © 2014 Pearson Education, Inc. Chapter 13

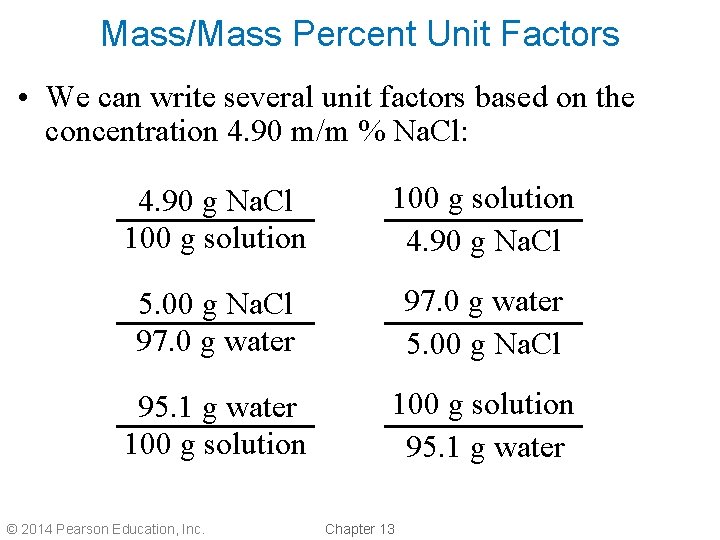
Mass/Mass Percent Unit Factors • We can write several unit factors based on the concentration 4. 90 m/m % Na. Cl: 4. 90 g Na. Cl 100 g solution 4. 90 g Na. Cl 5. 00 g Na. Cl 97. 0 g water 5. 00 g Na. Cl 95. 1 g water 100 g solution 95. 1 g water © 2014 Pearson Education, Inc. Chapter 13

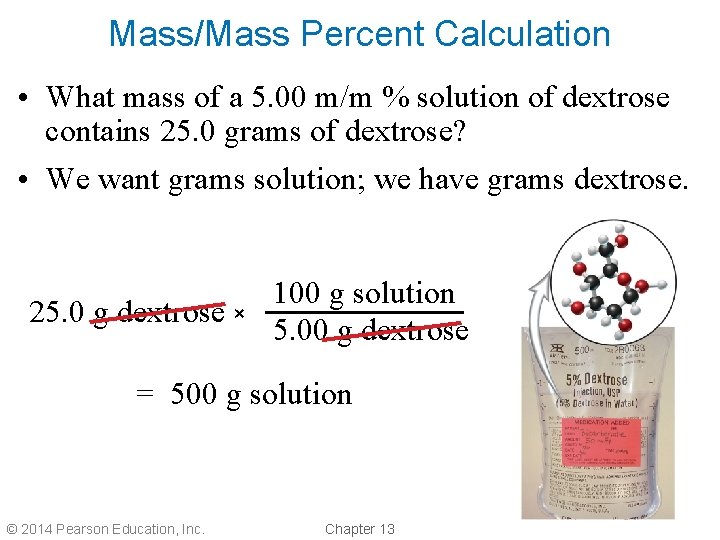
Mass/Mass Percent Calculation • What mass of a 5. 00 m/m % solution of dextrose contains 25. 0 grams of dextrose? • We want grams solution; we have grams dextrose. 25. 0 g dextrose × 100 g solution 5. 00 g dextrose = 500 g solution © 2014 Pearson Education, Inc. Chapter 13

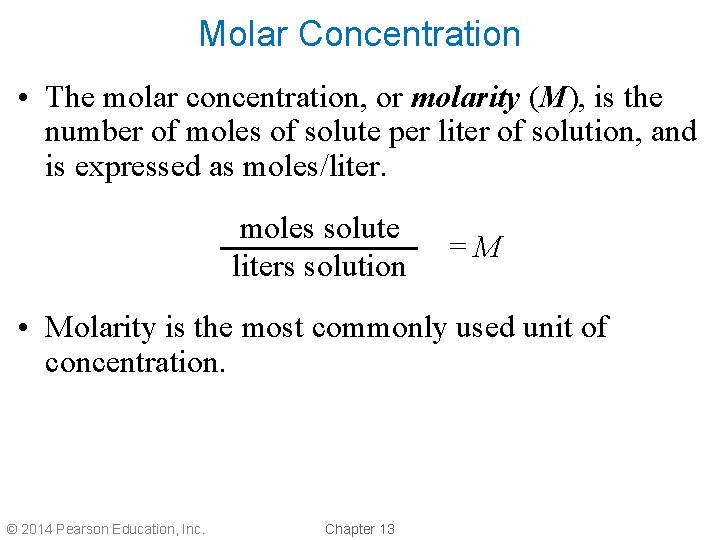
Molar Concentration • The molar concentration, or molarity (M), is the number of moles of solute per liter of solution, and is expressed as moles/liter. moles solute liters solution =M • Molarity is the most commonly used unit of concentration. © 2014 Pearson Education, Inc. Chapter 13

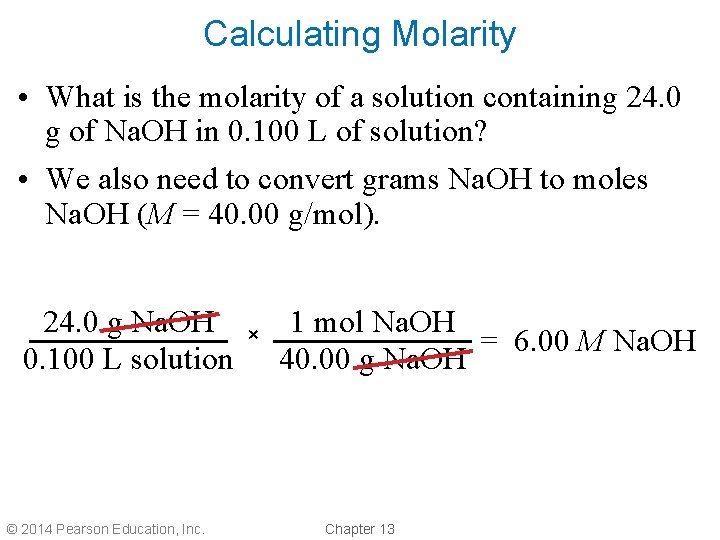
Calculating Molarity • What is the molarity of a solution containing 24. 0 g of Na. OH in 0. 100 L of solution? • We also need to convert grams Na. OH to moles Na. OH (M = 40. 00 g/mol). 24. 0 g Na. OH 0. 100 L solution © 2014 Pearson Education, Inc. × 1 mol Na. OH = 6. 00 M Na. OH 40. 00 g Na. OH Chapter 13

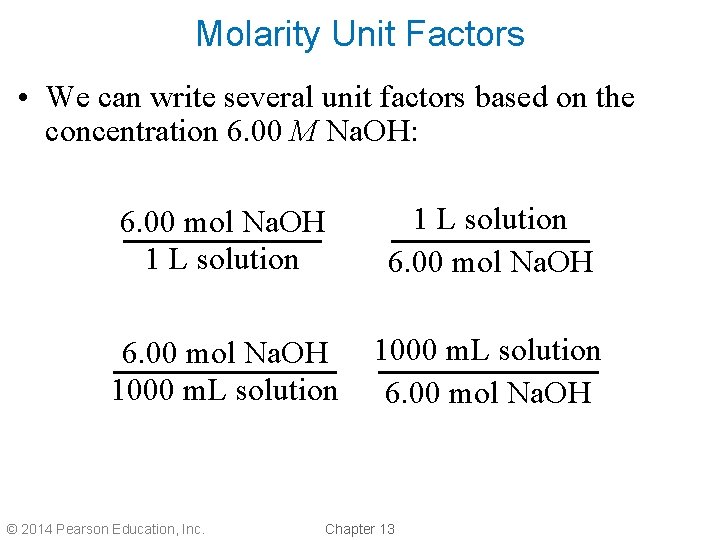
Molarity Unit Factors • We can write several unit factors based on the concentration 6. 00 M Na. OH: 6. 00 mol Na. OH 1 L solution 6. 00 mol Na. OH 1000 m. L solution 6. 00 mol Na. OH © 2014 Pearson Education, Inc. Chapter 13

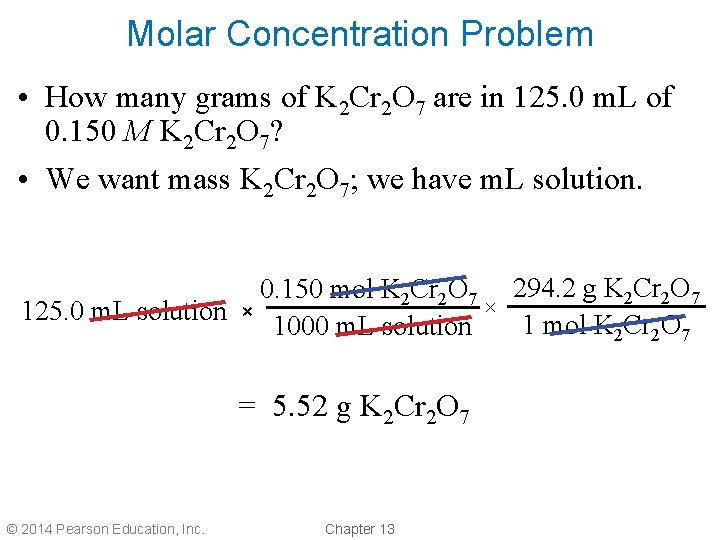
Molar Concentration Problem • How many grams of K 2 Cr 2 O 7 are in 125. 0 m. L of 0. 150 M K 2 Cr 2 O 7? • We want mass K 2 Cr 2 O 7; we have m. L solution. 0. 150 mol K 2 Cr 2 O 7 294. 2 g K 2 Cr 2 O 7 × 125. 0 m. L solution × 1 mol K 2 Cr 2 O 7 1000 m. L solution = 5. 52 g K 2 Cr 2 O 7 © 2014 Pearson Education, Inc. Chapter 13

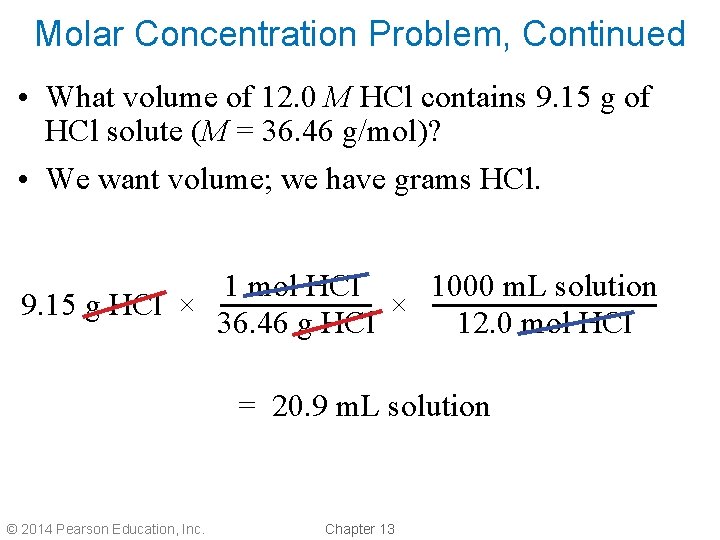
Molar Concentration Problem, Continued • What volume of 12. 0 M HCl contains 9. 15 g of HCl solute (M = 36. 46 g/mol)? • We want volume; we have grams HCl. 1 mol HCl 1000 m. L solution 9. 15 g HCl × × 36. 46 g HCl 12. 0 mol HCl = 20. 9 m. L solution © 2014 Pearson Education, Inc. Chapter 13

Critical Thinking: Water Fluoridation • Cities often add fluoride to drinking water. • Tooth enamel is made mostly of the mineral hydroxyapatite, Ca 10(PO 4)6(OH)2. • Fluoride prevents tooth decay by converting some of the hydroxyapatite to Ca 10(PO 4)6 F 2, which is more resistant to acid. • Typically, fluoridation levels are less than 1 mg/L. © 2014 Pearson Education, Inc. Chapter 13

Dilution of a Solution • Rather than prepare a solution by dissolving a solid in water, we can prepare a solution by diluting a more concentrated solution. • When performing a dilution, the amount of solute does not change, only the amount of solvent. • The equation we use is: M 1 × V 1 = M 2 × V 2. – M 1 and V 1 are the initial molarity and volume, and M 2 and V 2 are the new molarity and volume. © 2014 Pearson Education, Inc. Chapter 13

Dilution Problem • What volume of 6. 0 M Na. OH needs to be diluted to prepare 5. 00 L if 0. 10 M Na. OH? • We want final volume and we have our final volume and concentration. M 1 × V 1 = M 2 × V 2 (6. 0 M) × V 1 = (0. 10 M) × (5. 00 L) V 1 = = 0. 083 L 6. 0 M © 2014 Pearson Education, Inc. Chapter 13

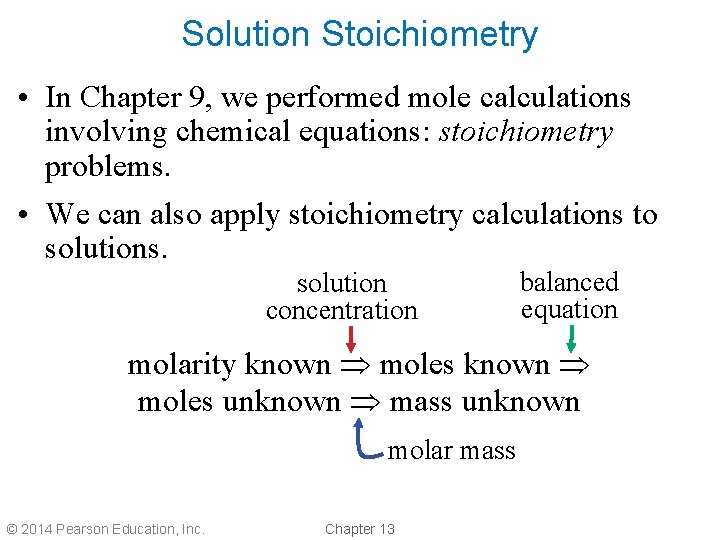
Solution Stoichiometry • In Chapter 9, we performed mole calculations involving chemical equations: stoichiometry problems. • We can also apply stoichiometry calculations to solutions. solution concentration balanced equation molarity known moles unknown mass unknown molar mass © 2014 Pearson Education, Inc. Chapter 13

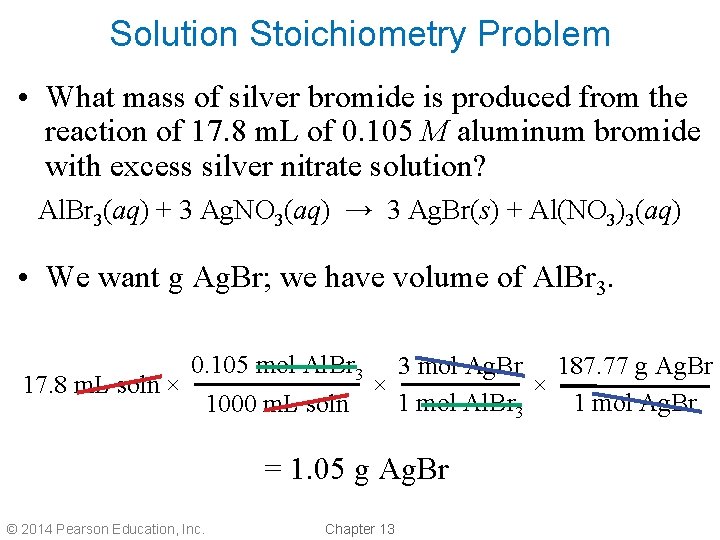
Solution Stoichiometry Problem • What mass of silver bromide is produced from the reaction of 17. 8 m. L of 0. 105 M aluminum bromide with excess silver nitrate solution? Al. Br 3(aq) + 3 Ag. NO 3(aq) → 3 Ag. Br(s) + Al(NO 3)3(aq) • We want g Ag. Br; we have volume of Al. Br 3. 0. 105 mol Al. Br 3 3 mol Ag. Br 187. 77 g Ag. Br 17. 8 m. L soln × × × 1 mol Ag. Br 1 mol Al. Br 3 1000 m. L soln = 1. 05 g Ag. Br © 2014 Pearson Education, Inc. Chapter 13

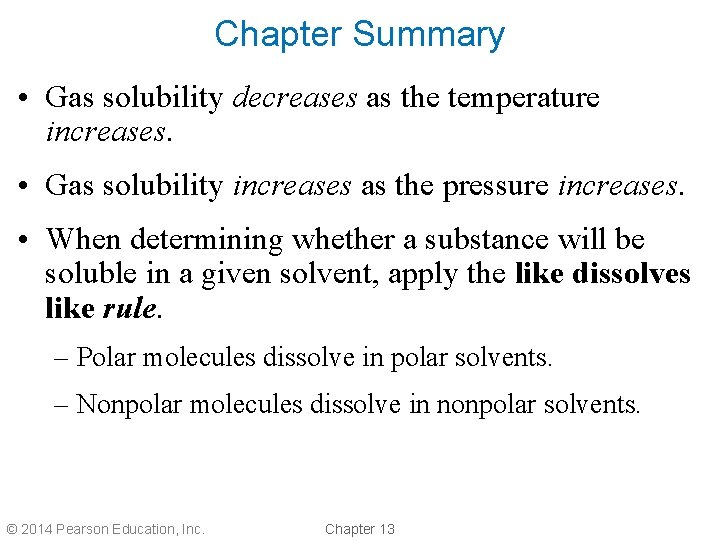
Chapter Summary • Gas solubility decreases as the temperature increases. • Gas solubility increases as the pressure increases. • When determining whether a substance will be soluble in a given solvent, apply the like dissolves like rule. – Polar molecules dissolve in polar solvents. – Nonpolar molecules dissolve in nonpolar solvents. © 2014 Pearson Education, Inc. Chapter 13

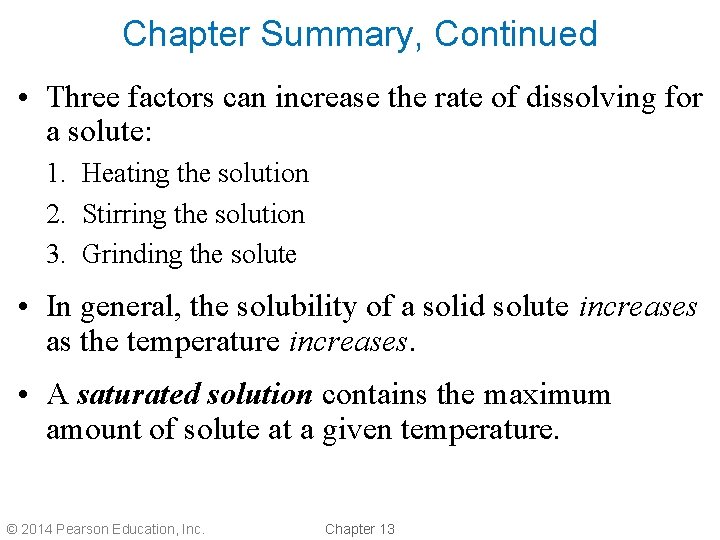
Chapter Summary, Continued • Three factors can increase the rate of dissolving for a solute: 1. Heating the solution 2. Stirring the solution 3. Grinding the solute • In general, the solubility of a solid solute increases as the temperature increases. • A saturated solution contains the maximum amount of solute at a given temperature. © 2014 Pearson Education, Inc. Chapter 13

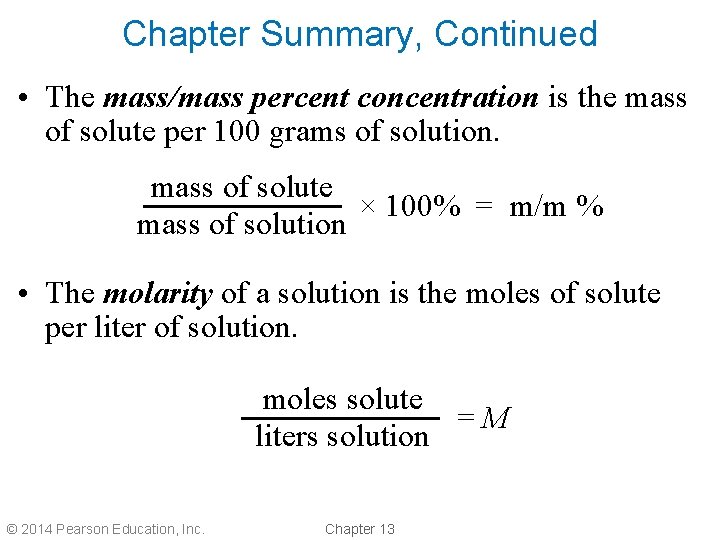
Chapter Summary, Continued • The mass/mass percent concentration is the mass of solute per 100 grams of solution. mass of solute × 100% = m/m % mass of solution • The molarity of a solution is the moles of solute per liter of solution. moles solute =M liters solution © 2014 Pearson Education, Inc. Chapter 13

Chapter Summary, Continued • You can make a solution by diluting a more concentrated solution. M 1 × V 1 = M 2 × V 2 • We can apply stoichiometry to reactions involving solutions using the molarity as a unit factor to convert between moles and volume. © 2014 Pearson Education, Inc. Chapter 13