Chapter 6 Copper and Copper Alloys MS 371


- Slides: 23

Chapter 6 Copper and Copper Alloys /MS 371/ Structure and Properties of Engineering Alloys

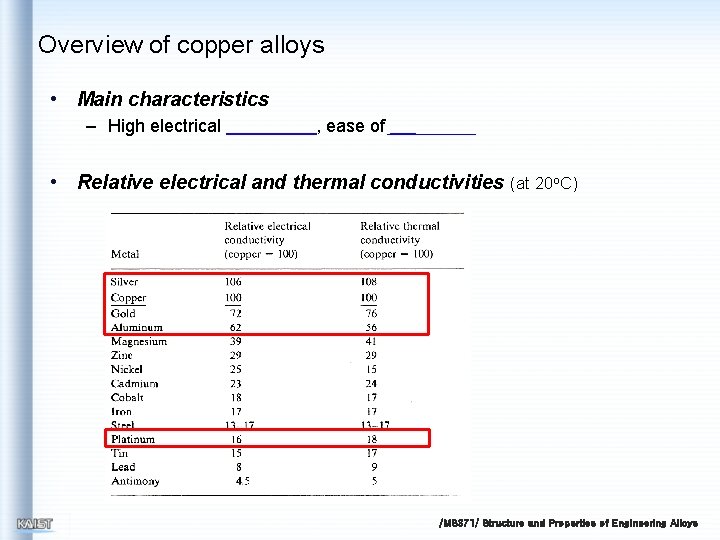
Overview of copper alloys • Main characteristics – High electrical , ease of • Relative electrical and thermal conductivities (at 20 o. C) /MS 371/ Structure and Properties of Engineering Alloys

Overview of copper alloys • Cu + Zn (黃銅) : good ! – high Zn, high strength: edge of stairs, coin – small damping capacity: instrument (징) • Cu + Sn (靑銅) : good – resistance statue • Cu + Ni : 100圓, 500원 • Cu + Pb : good mechanical work • Cu + Be : material of (no spark, not such as flint or firestone) /MS 371/ Structure and Properties of Engineering Alloys

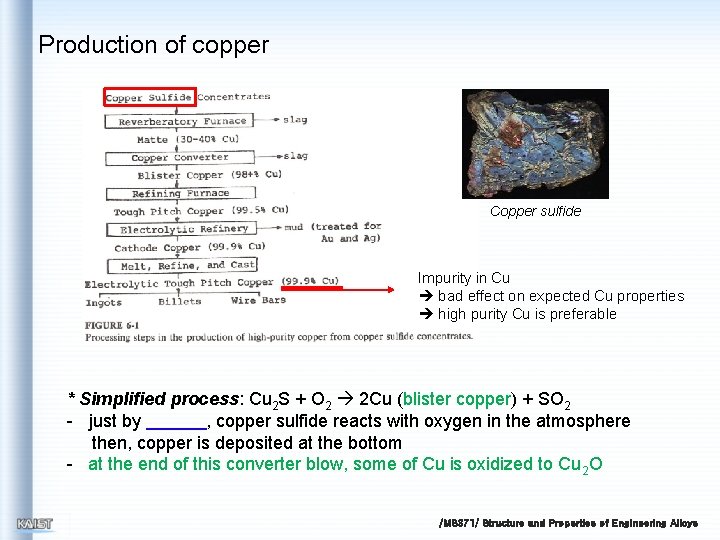
Production of copper Copper sulfide Impurity in Cu bad effect on expected Cu properties high purity Cu is preferable * Simplified process: Cu 2 S + O 2 2 Cu (blister copper) + SO 2 - just by , copper sulfide reacts with oxygen in the atmosphere then, copper is deposited at the bottom - at the end of this converter blow, some of Cu is oxidized to Cu 2 O /MS 371/ Structure and Properties of Engineering Alloys

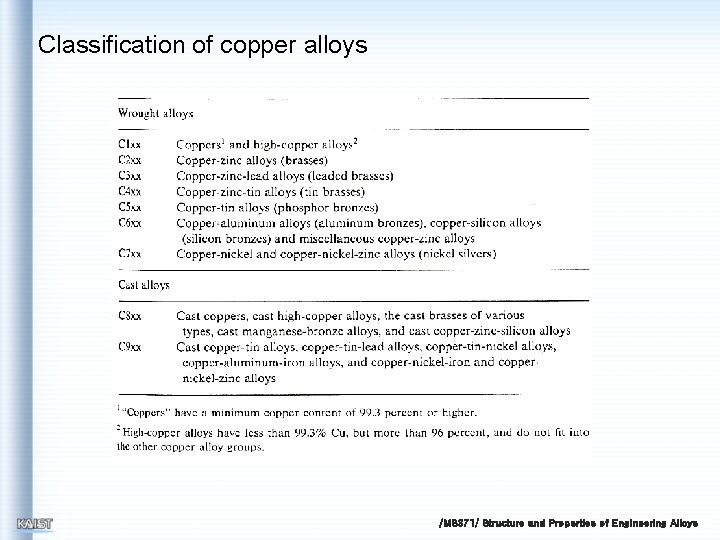
Classification of copper alloys /MS 371/ Structure and Properties of Engineering Alloys

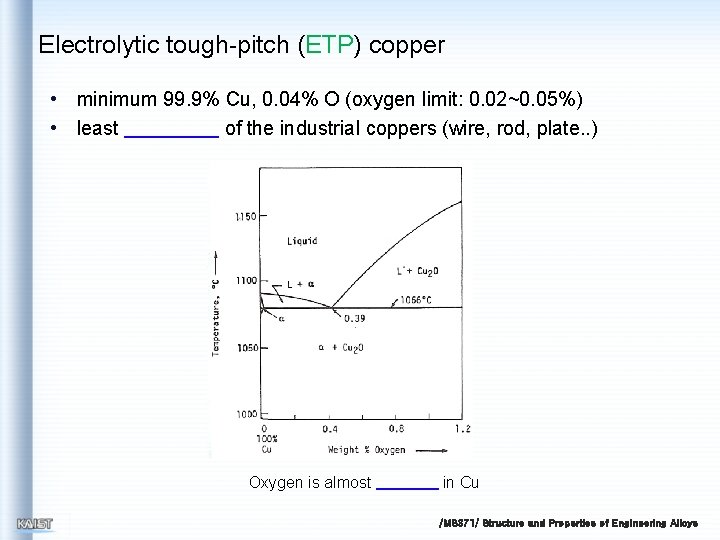
Electrolytic tough-pitch (ETP) copper • minimum 99. 9% Cu, 0. 04% O (oxygen limit: 0. 02~0. 05%) • least of the industrial coppers (wire, rod, plate. . ) Oxygen is almost in Cu /MS 371/ Structure and Properties of Engineering Alloys

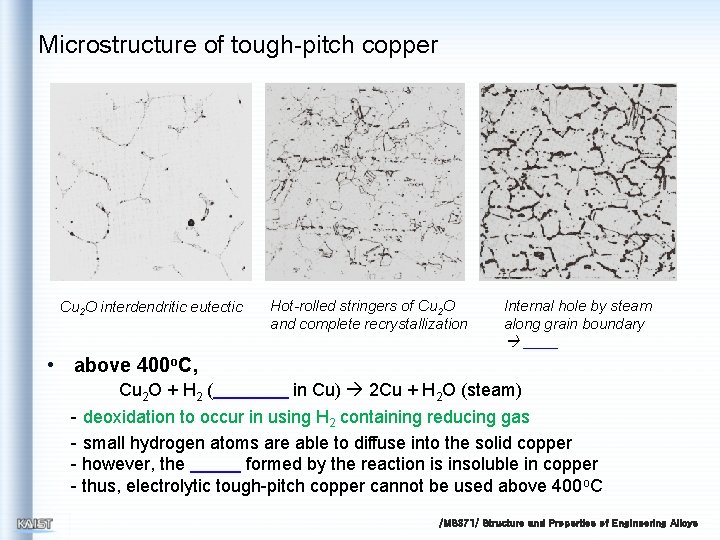
Microstructure of tough-pitch copper Cu 2 O interdendritic eutectic Hot-rolled stringers of Cu 2 O and complete recrystallization Internal hole by steam along grain boundary • above 400 o. C, Cu 2 O + H 2 ( in Cu) 2 Cu + H 2 O (steam) - deoxidation to occur in using H 2 containing reducing gas - small hydrogen atoms are able to diffuse into the solid copper - however, the formed by the reaction is insoluble in copper - thus, electrolytic tough-pitch copper cannot be used above 400 o. C /MS 371/ Structure and Properties of Engineering Alloys

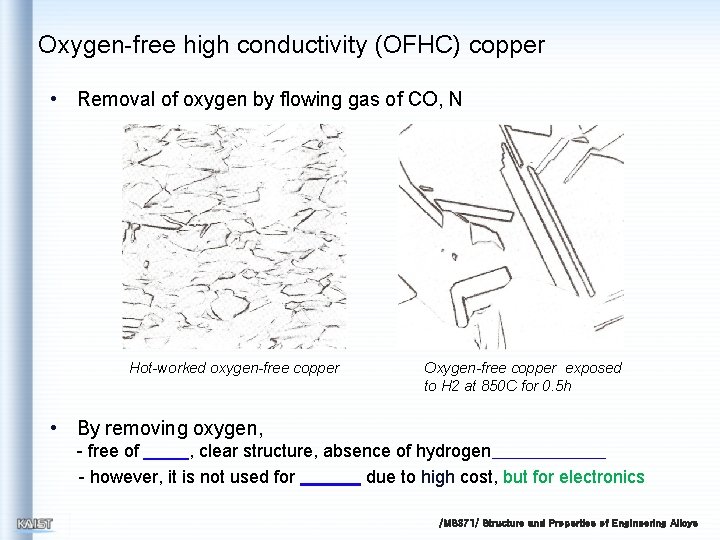
Oxygen-free high conductivity (OFHC) copper • Removal of oxygen by flowing gas of CO, N Hot-worked oxygen-free copper Oxygen-free copper exposed to H 2 at 850 C for 0. 5 h • By removing oxygen, - free of , clear structure, absence of hydrogen - however, it is not used for due to high cost, but for electronics /MS 371/ Structure and Properties of Engineering Alloys

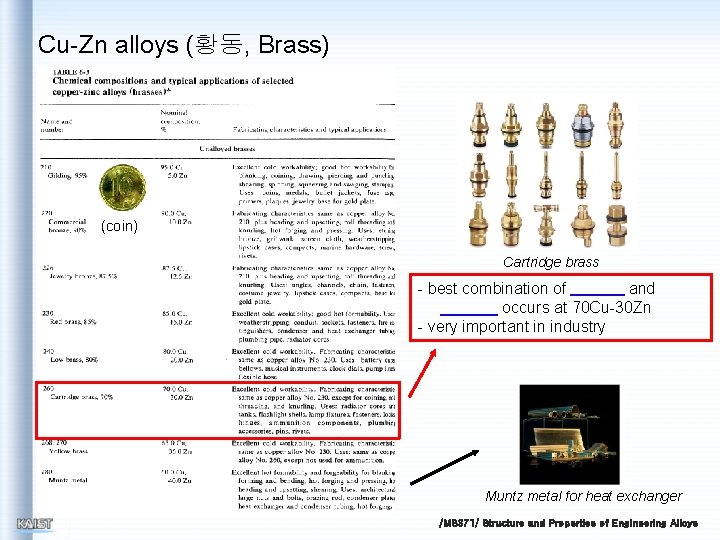
Cu-Zn alloys (황동, Brass) (coin) Cartridge brass - best combination of and occurs at 70 Cu-30 Zn - very important in industry Muntz metal for heat exchanger /MS 371/ Structure and Properties of Engineering Alloys

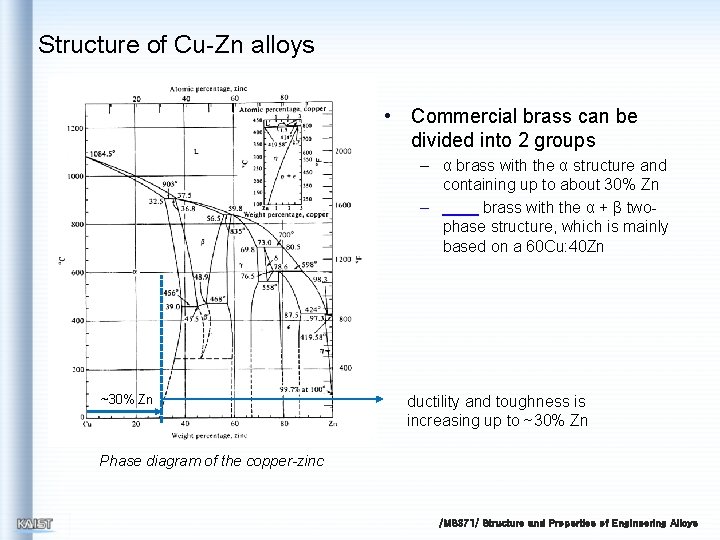
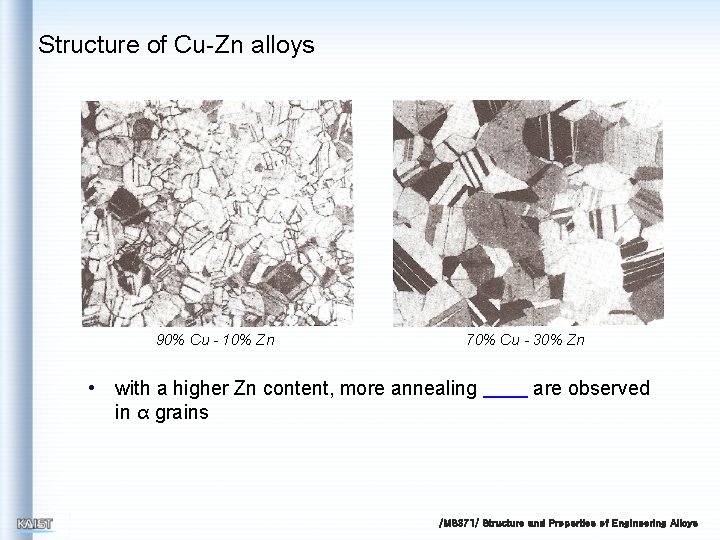
Structure of Cu-Zn alloys • Commercial brass can be divided into 2 groups – α brass with the α structure and containing up to about 30% Zn – brass with the α + β twophase structure, which is mainly based on a 60 Cu: 40 Zn ~30% Zn ductility and toughness is increasing up to ~30% Zn Phase diagram of the copper-zinc /MS 371/ Structure and Properties of Engineering Alloys

Structure of Cu-Zn alloys 90% Cu - 10% Zn 70% Cu - 30% Zn • with a higher Zn content, more annealing in α grains are observed /MS 371/ Structure and Properties of Engineering Alloys

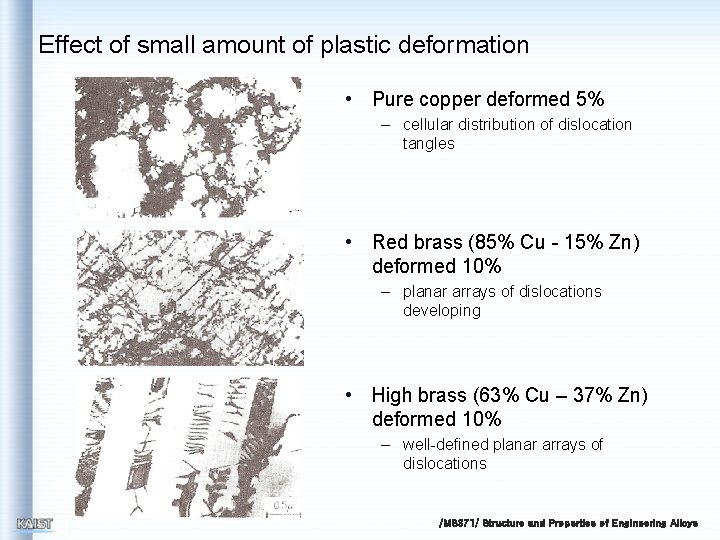
Effect of small amount of plastic deformation • Pure copper deformed 5% – cellular distribution of dislocation tangles • Red brass (85% Cu - 15% Zn) deformed 10% – planar arrays of dislocations developing • High brass (63% Cu – 37% Zn) deformed 10% – well-defined planar arrays of dislocations /MS 371/ Structure and Properties of Engineering Alloys

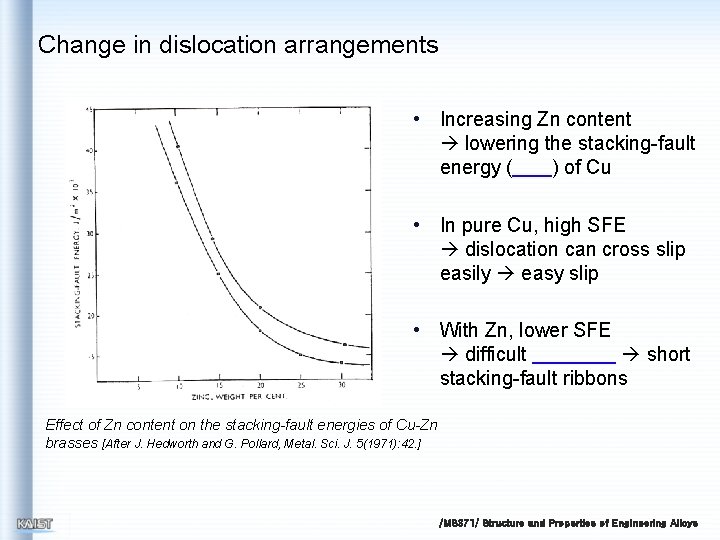
Change in dislocation arrangements • Increasing Zn content lowering the stacking-fault energy ( ) of Cu • In pure Cu, high SFE dislocation can cross slip easily easy slip • With Zn, lower SFE difficult short stacking-fault ribbons Effect of Zn content on the stacking-fault energies of Cu-Zn brasses [After J. Hedworth and G. Pollard, Metal. Sci. J. 5(1971): 42. ] /MS 371/ Structure and Properties of Engineering Alloys

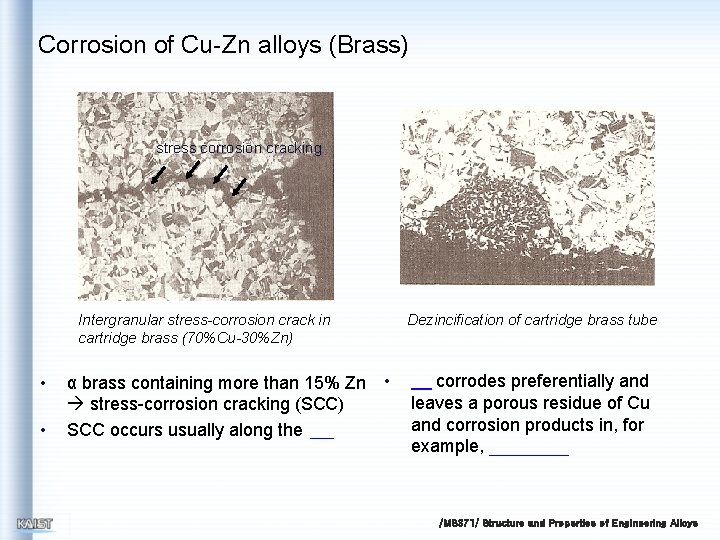
Corrosion of Cu-Zn alloys (Brass) stress corrosion cracking Intergranular stress-corrosion crack in cartridge brass (70%Cu-30%Zn) • • α brass containing more than 15% Zn • stress-corrosion cracking (SCC) SCC occurs usually along the Dezincification of cartridge brass tube corrodes preferentially and leaves a porous residue of Cu and corrosion products in, for example, /MS 371/ Structure and Properties of Engineering Alloys

Cu-Sn alloys (청동, Bronze) • ‘Sn’ addition to prevent ; ‘tin bronze’ • ‘P’ addition as a agent during casting; ‘phosphor bronzes’ • High strength, wear resistance, good sea-water corrosion resistance Comparison of purpose Brass Bronze Good formability Excellent corrosion resistance /MS 371/ Structure and Properties of Engineering Alloys

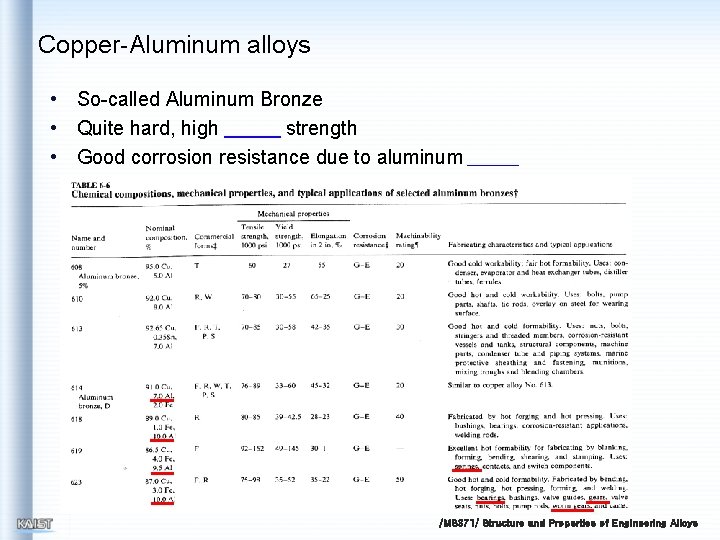
Copper-Aluminum alloys • So-called Aluminum Bronze • Quite hard, high strength • Good corrosion resistance due to aluminum /MS 371/ Structure and Properties of Engineering Alloys

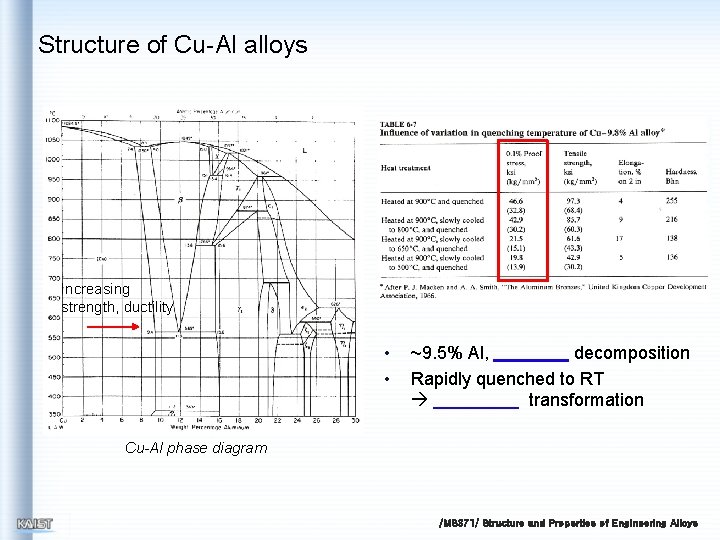
Structure of Cu-Al alloys Increasing strength, ductility • • ~9. 5% Al, decomposition Rapidly quenched to RT transformation Cu-Al phase diagram /MS 371/ Structure and Properties of Engineering Alloys

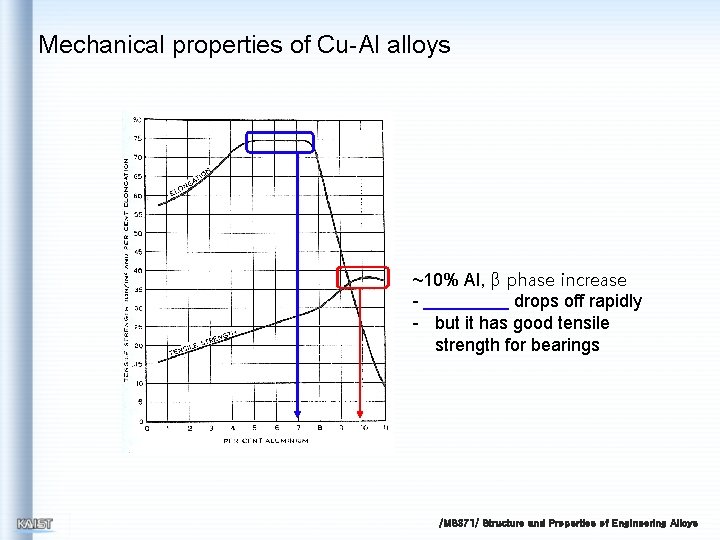
Mechanical properties of Cu-Al alloys ~10% Al, β phase increase drops off rapidly - but it has good tensile strength for bearings /MS 371/ Structure and Properties of Engineering Alloys

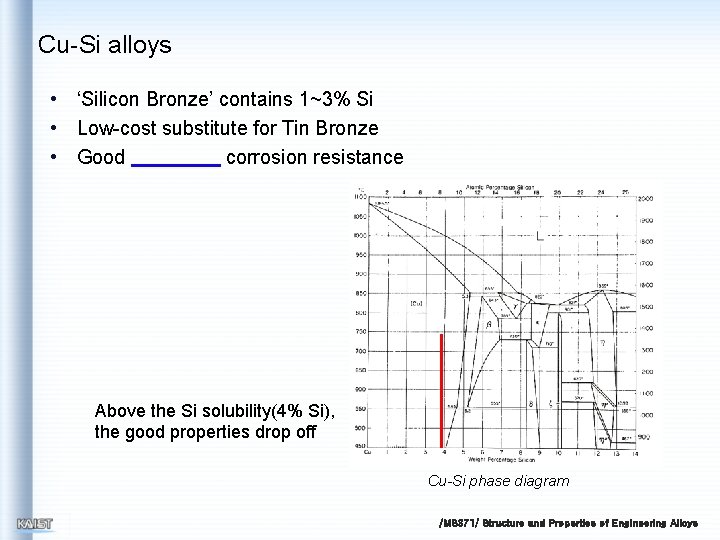
Cu-Si alloys • ‘Silicon Bronze’ contains 1~3% Si • Low-cost substitute for Tin Bronze • Good corrosion resistance Above the Si solubility(4% Si), the good properties drop off Cu-Si phase diagram /MS 371/ Structure and Properties of Engineering Alloys

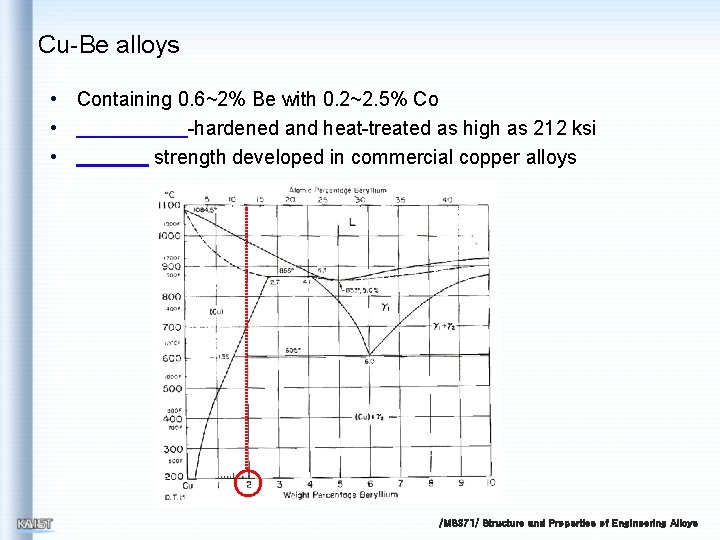
Cu-Be alloys • Containing 0. 6~2% Be with 0. 2~2. 5% Co • -hardened and heat-treated as high as 212 ksi • strength developed in commercial copper alloys /MS 371/ Structure and Properties of Engineering Alloys

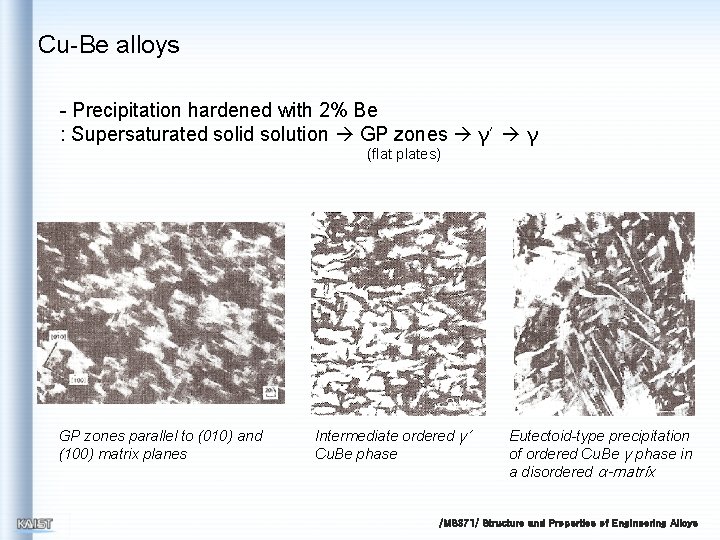
Cu-Be alloys - Precipitation hardened with 2% Be : Supersaturated solid solution GP zones γ’ γ (flat plates) GP zones parallel to (010) and (100) matrix planes Intermediate ordered γ’ Cu. Be phase Eutectoid-type precipitation of ordered Cu. Be γ phase in a disordered α-matrix /MS 371/ Structure and Properties of Engineering Alloys

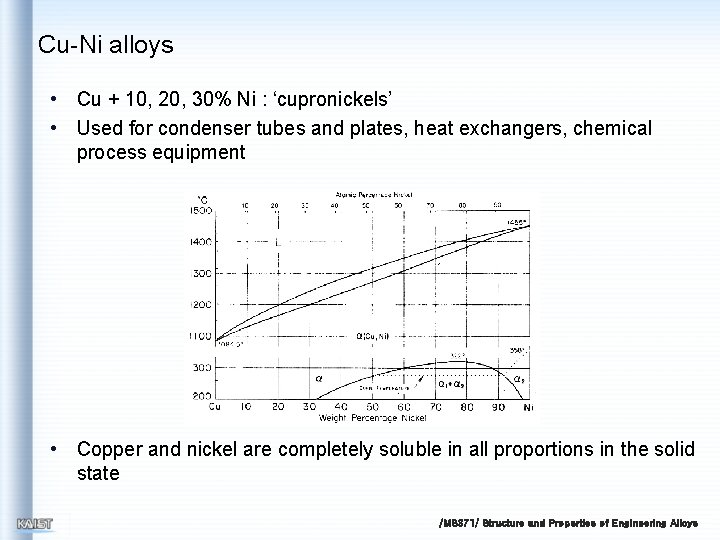
Cu-Ni alloys • Cu + 10, 20, 30% Ni : ‘cupronickels’ • Used for condenser tubes and plates, heat exchangers, chemical process equipment • Copper and nickel are completely soluble in all proportions in the solid state /MS 371/ Structure and Properties of Engineering Alloys

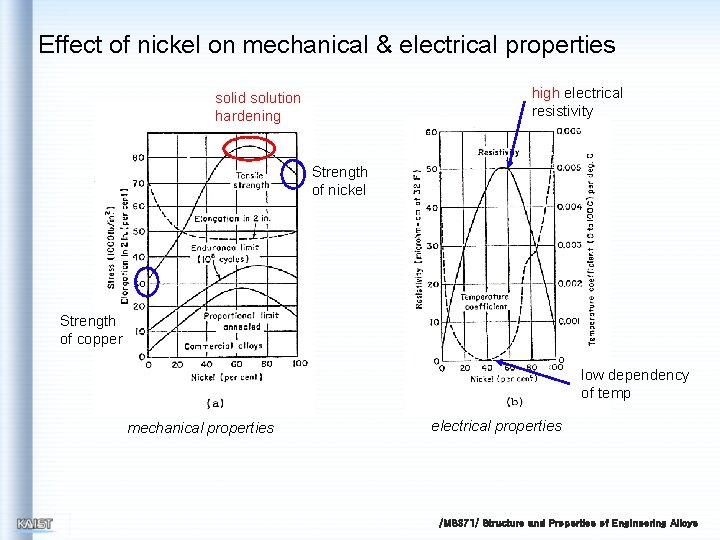
Effect of nickel on mechanical & electrical properties high electrical resistivity solid solution hardening Strength of nickel Strength of copper low dependency of temp mechanical properties electrical properties /MS 371/ Structure and Properties of Engineering Alloys