Chapter 4 reaction in aqueous solution 4 5


- Slides: 13

Chapter 4 reaction in aqueous solution � 4. 5 v Concentration of solutions dilution of solutions Ø Homework Page 163→ 4 -60, 4 -62, 4 -64 , 4 -66 , 4 -70 , 4 -74

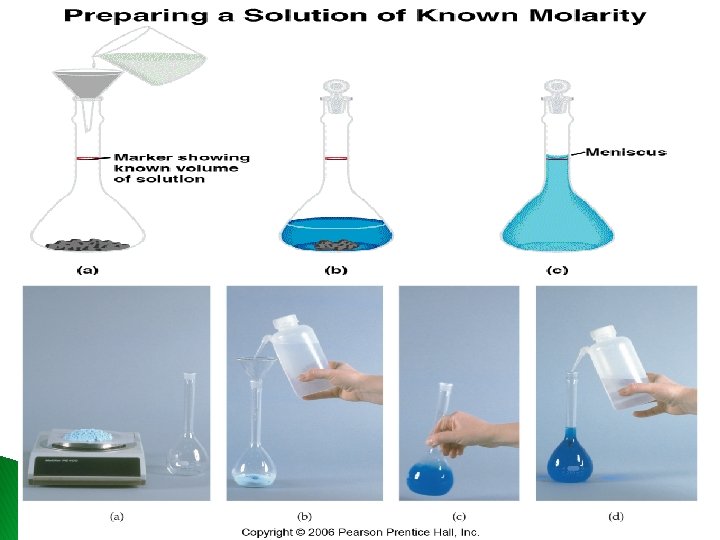
4. 5 Concentration of solutions ü The concentration of a solution is the amount of solute present in a given amount of solvent , it can be expressed in terms of its molarity (molar concentration) moles of solute Molarity (M) = volume of solution in liters Have mol and vol molarity � Have molarity and vol mol of solute � Have molarity and mol of solute volume � AND: mol of solute grams of solute �


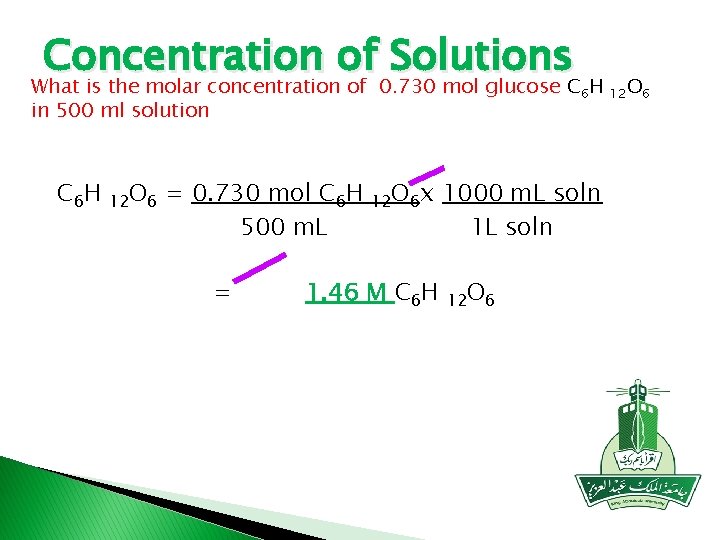
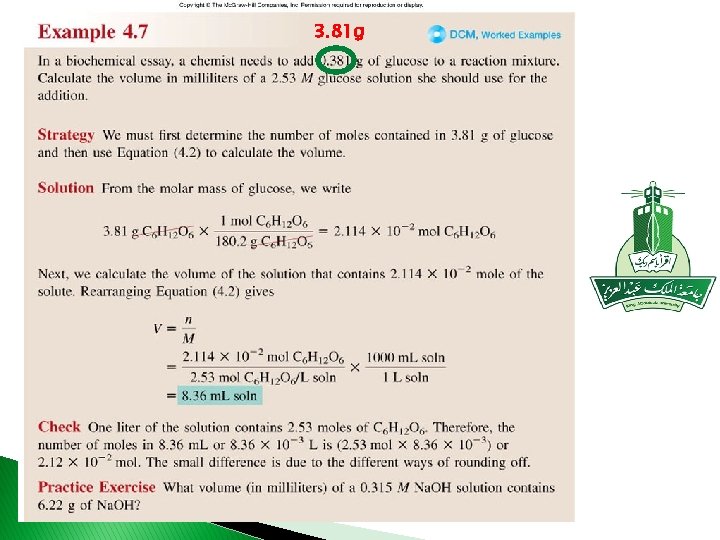
Concentration of Solutions What is the molar concentration of 0. 730 mol glucose C 6 H in 500 ml solution C 6 H 12 O 6 = 0. 730 mol C 6 H 500 m. L = 12 O 6 x 1. 46 M C 6 H 1000 m. L soln 12 O 6


3. 81 g

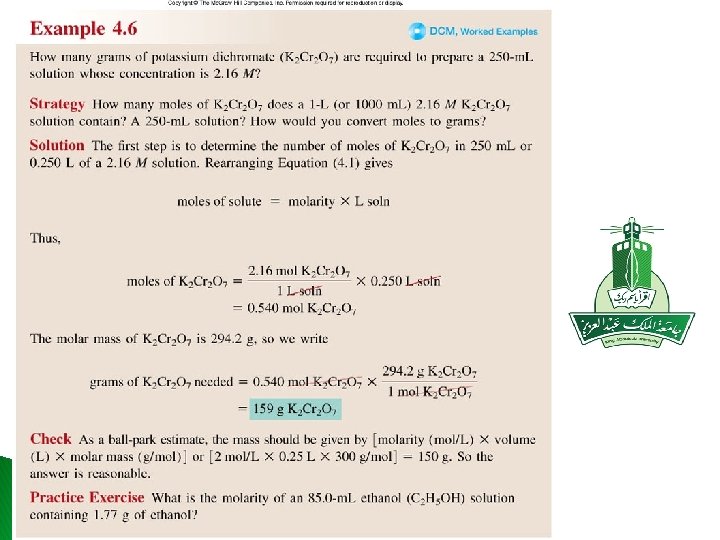
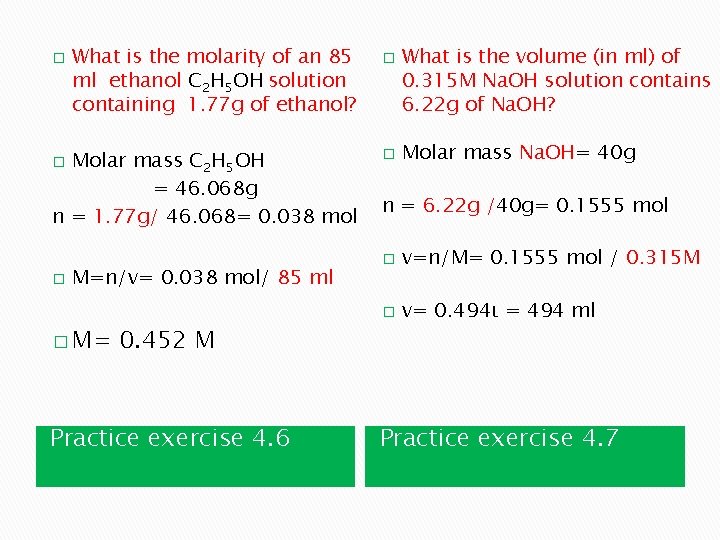
� What is the molarity of an 85 ml ethanol C 2 H 5 OH solution containing 1. 77 g of ethanol? Molar mass C 2 H 5 OH = 46. 068 g n = 1. 77 g/ 46. 068= 0. 038 mol � � M=n/v= 0. 038 mol/ 85 ml � M= 0. 452 M Practice exercise 4. 6 � � What is the volume (in ml) of 0. 315 M Na. OH solution contains 6. 22 g of Na. OH? Molar mass Na. OH= 40 g n = 6. 22 g /40 g= 0. 1555 mol � v=n/M= 0. 1555 mol / 0. 315 M � v= 0. 494ι = 494 ml Practice exercise 4. 7

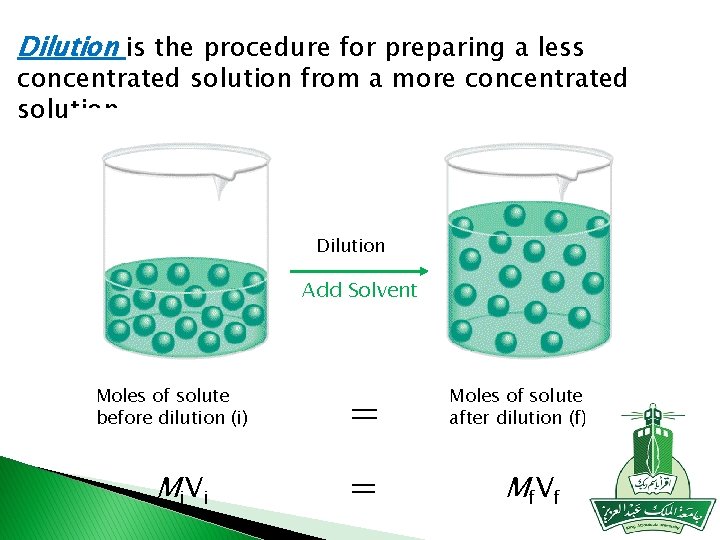
Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent Moles of solute before dilution (i) M i. V i = = Moles of solute after dilution (f) Mf V f

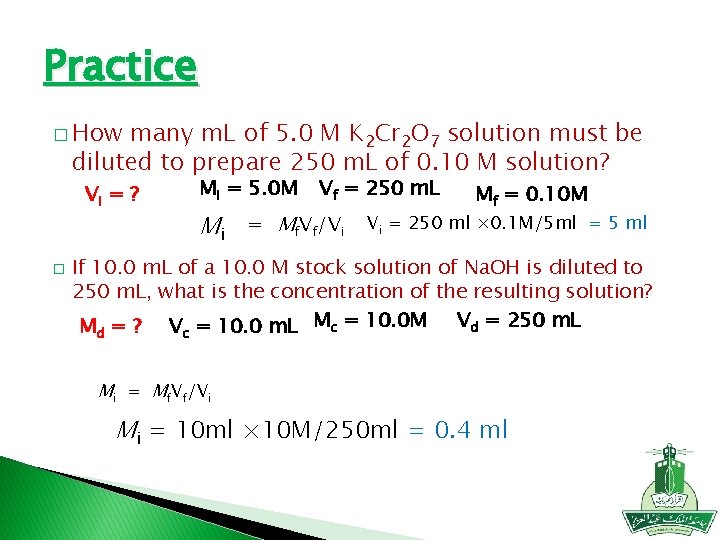
Practice � How many m. L of 5. 0 M K 2 Cr 2 O 7 solution must be diluted to prepare 250 m. L of 0. 10 M solution? Mi = 5. 0 M Vi = ? � Mi Vf = 250 m. L = Mf. Vf/Vi Mf = 0. 10 M Vi = 250 ml × 0. 1 M/5 ml = 5 ml If 10. 0 m. L of a 10. 0 M stock solution of Na. OH is diluted to 250 m. L, what is the concentration of the resulting solution? Vd = 250 m. L M = ? V = 10. 0 m. L Mc = 10. 0 M d c Mi = Mf. Vf/Vi Mi = 10 ml × 10 M/250 ml = 0. 4 ml

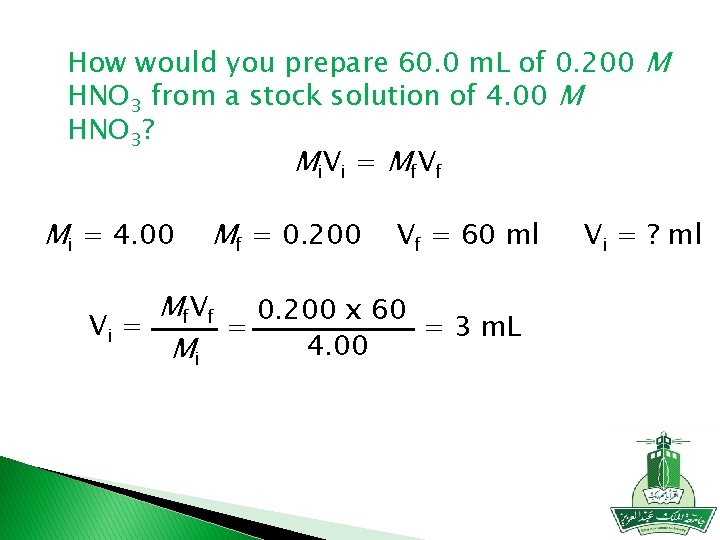
How would you prepare 60. 0 m. L of 0. 200 M HNO 3 from a stock solution of 4. 00 M HNO 3? M i. V i = M f V f Mi = 4. 00 Mf = 0. 200 Vf = 60 ml Mf. Vf 0. 200 x 60 Vi = = 3 m. L = 4. 00 Mi Vi = ? ml


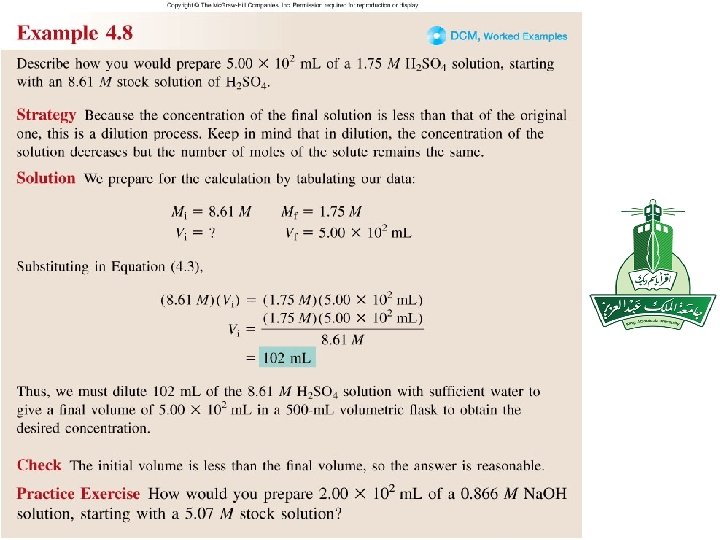
Practice exercise 4. 9 How would you prepare 200 m. L of 0. 866 M Na. OH from a stock solution of 5. 07 M Na. OH? M i. V i = M f V f Mi = 5. 07 Mf = 0. 866 Vf = 200 ml Vi = ? ml Mf. Vf 0. 866 x 200 Vi = = 34. 2 m. L = 5. 07 Mi

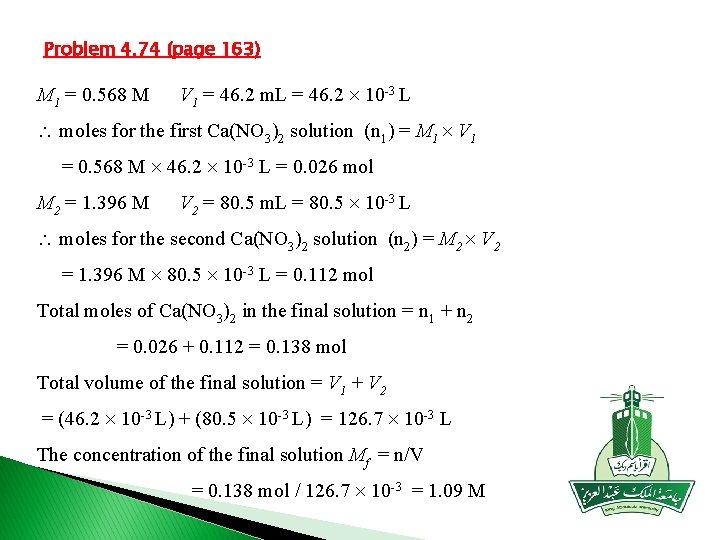
Problem 4. 74 (page 163) M 1 = 0. 568 M V 1 = 46. 2 m. L = 46. 2 10 -3 L moles for the first Ca(NO 3)2 solution (n 1) = M 1 V 1 = 0. 568 M 46. 2 10 -3 L = 0. 026 mol M 2 = 1. 396 M V 2 = 80. 5 m. L = 80. 5 10 -3 L moles for the second Ca(NO 3)2 solution (n 2) = M 2 V 2 = 1. 396 M 80. 5 10 -3 L = 0. 112 mol Total moles of Ca(NO 3)2 in the final solution = n 1 + n 2 = 0. 026 + 0. 112 = 0. 138 mol Total volume of the final solution = V 1 + V 2 = (46. 2 10 -3 L) + (80. 5 10 -3 L) = 126. 7 10 -3 L The concentration of the final solution Mf = n/V = 0. 138 mol / 126. 7 10 -3 = 1. 09 M