Chapter 4 Flowing Fluids Pressure Variation part 2






- Slides: 18

Chapter 4: Flowing Fluids & Pressure Variation (part 2) Review visualizations Frames of reference (part 1) Euler’s equation of motion

Quick review of visualizations • Pathline - follows path of a single “fluid particle” – one particle – one starting point – several times • Streakline - connecting the dots from several particles passing the same point at different times – several particles – one starting point – one time • Streamline - tangent to the velocity – – several particles starting point irrelevant tangent to velocity one time • All three can be useful (depending on the flow) • Pathlines, streamlines, streaklines are the same in steady flows.

Two helpful distinctions • Laminar vs. turbulent flow • Lagrangian vs. Eulerian descriptions – Lagrangian: follow particle – Eulerian: measure local velocities, etc.

Quantitative description of motion • How do we quantitatively describe moton … of a solid particle? ? … of a fluid particle? ?

First pressure review (under pressure? ) • How can we find pressure in a fluid: …. . in a gas? ? …. . in a liquid? ? Let’s start by answering this for static fluids.

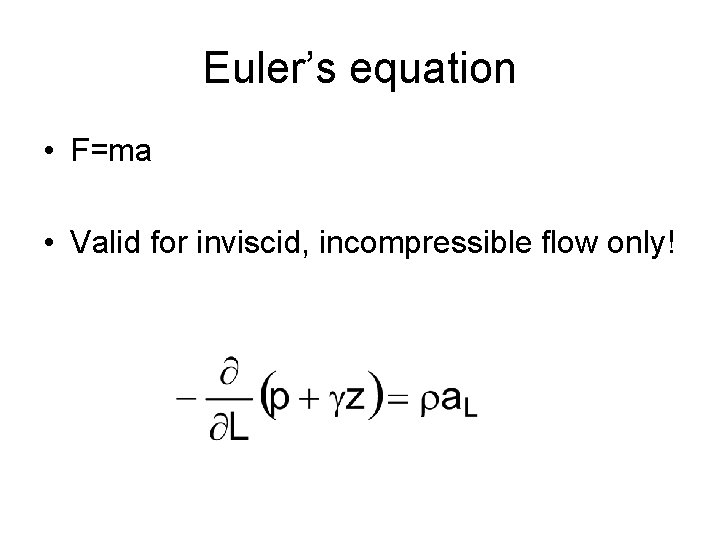
Euler’s equation • F=ma • Valid for inviscid, incompressible flow only!

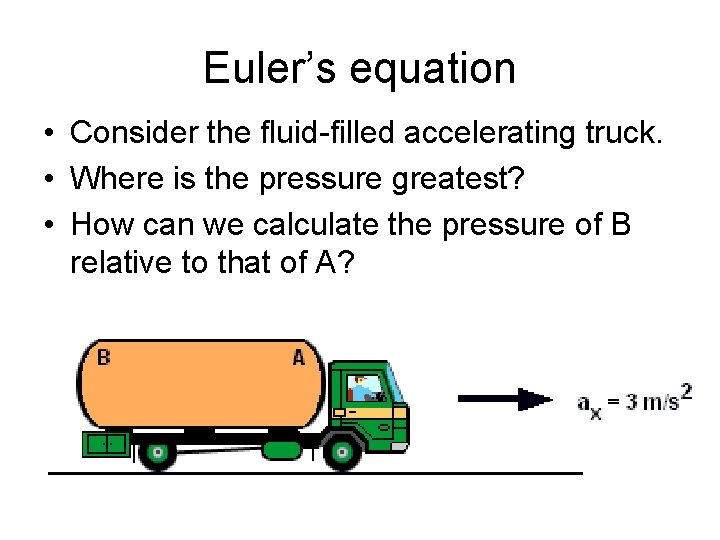
Euler’s equation • Consider the fluid-filled accelerating truck. • Where is the pressure greatest? • How can we calculate the pressure of B relative to that of A?

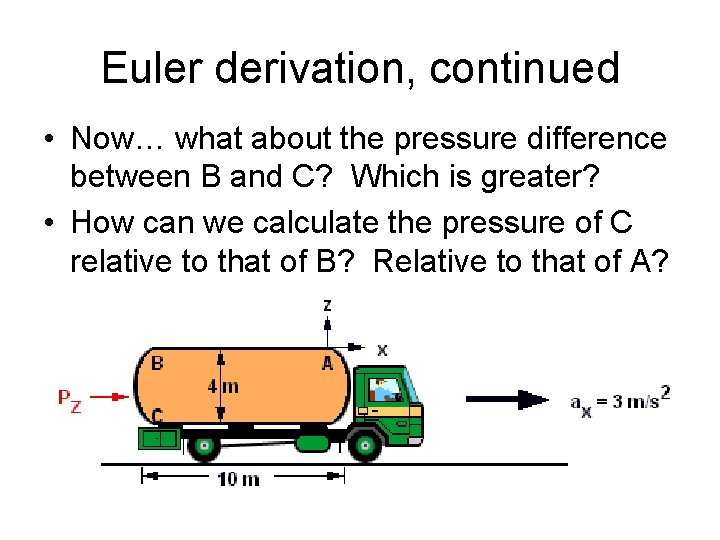
Euler derivation, continued • Now… what about the pressure difference between B and C? Which is greater? • How can we calculate the pressure of C relative to that of B? Relative to that of A?

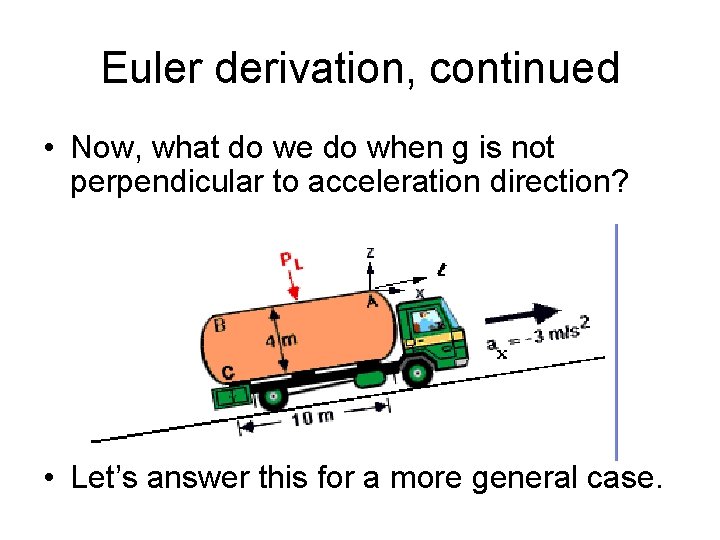
Euler derivation, continued • Now, what do we do when g is not perpendicular to acceleration direction? • Let’s answer this for a more general case.

Euler’s equation • F=ma • Valid for inviscid, incompressible flow only!

Chapter 4: Flowing Fluids & Pressure Variation (part 3) Euler’s equation of motion review Bernoulli’s equation of motion Types of fluid motion (part 2) Rotational motion

Bernoulli’s equation • F=ma along a streamline • Steady flow assumption required

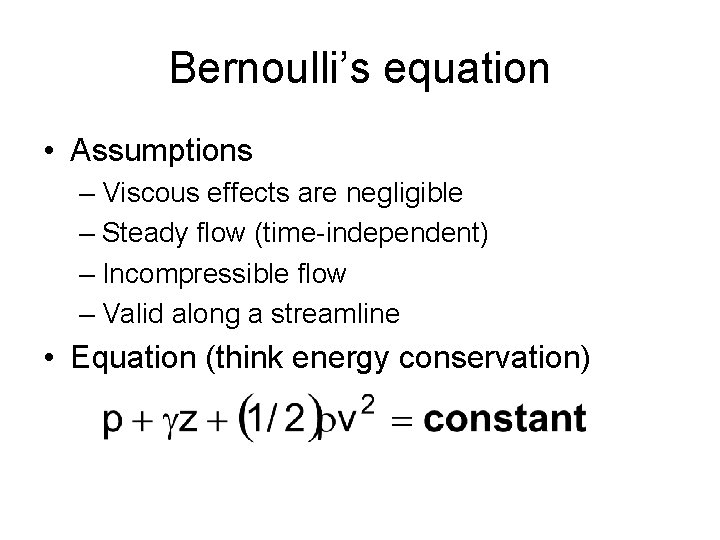
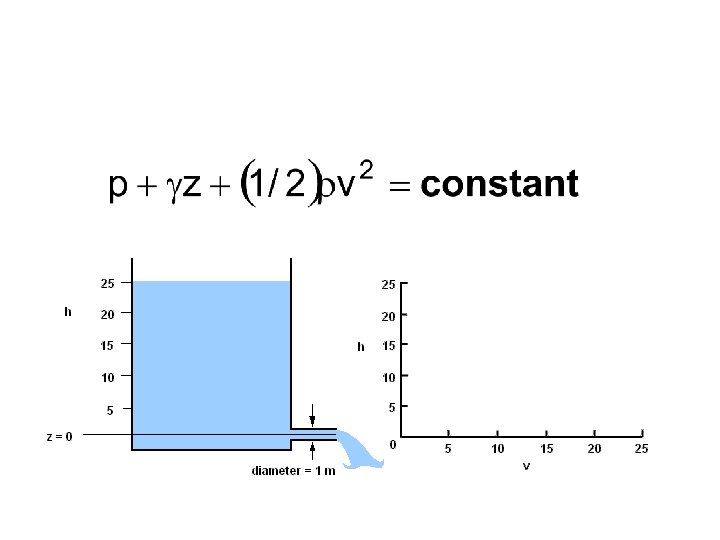
Bernoulli’s equation • Assumptions – Viscous effects are negligible – Steady flow (time-independent) – Incompressible flow – Valid along a streamline • Equation (think energy conservation)

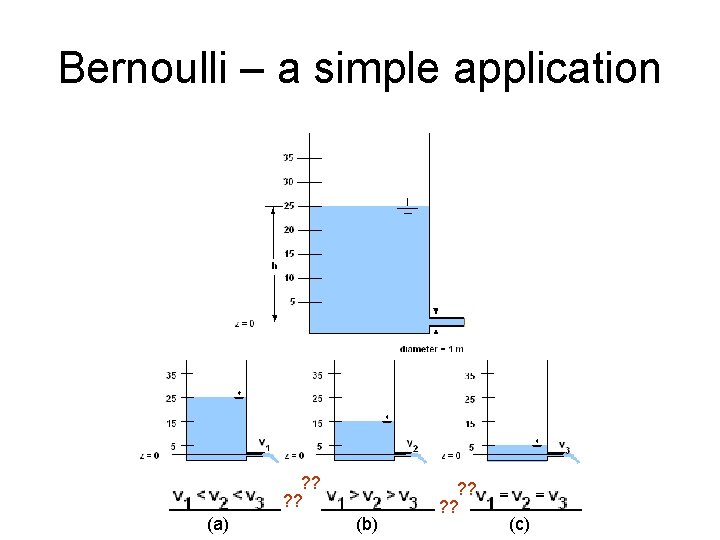
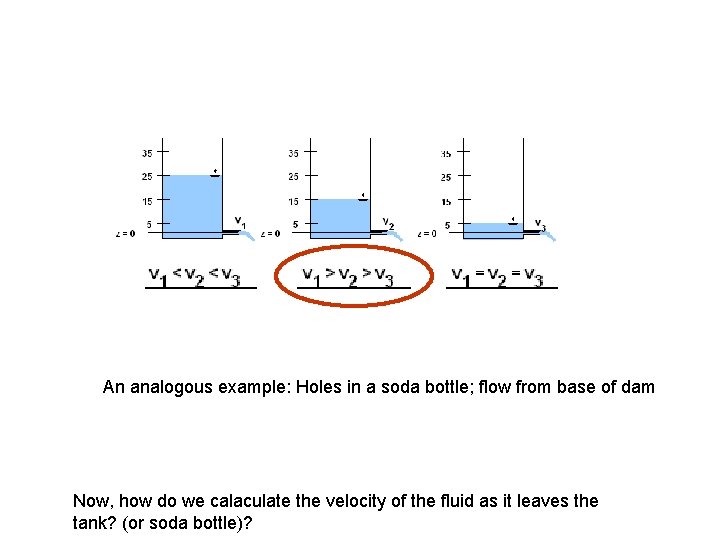
Bernoulli – a simple application ? ? (a) (b) ? ? (c)

An analogous example: Holes in a soda bottle; flow from base of dam Now, how do we calaculate the velocity of the fluid as it leaves the tank? (or soda bottle)?


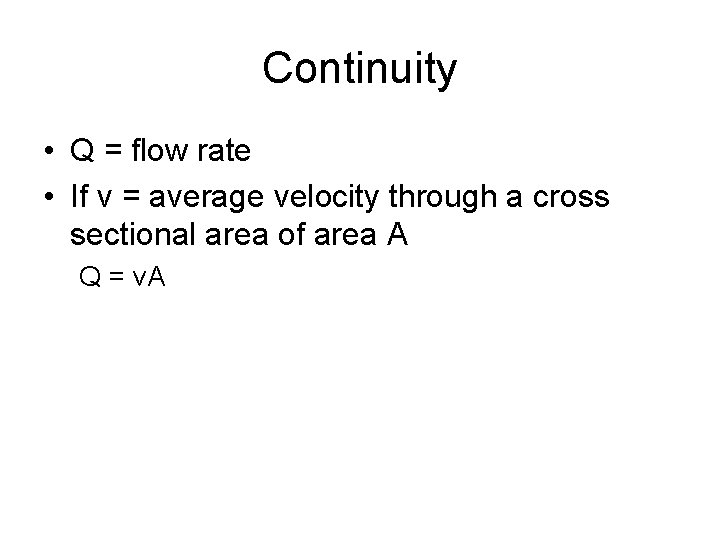
Continuity • Q = flow rate • If v = average velocity through a cross sectional area of area A Q = v. A

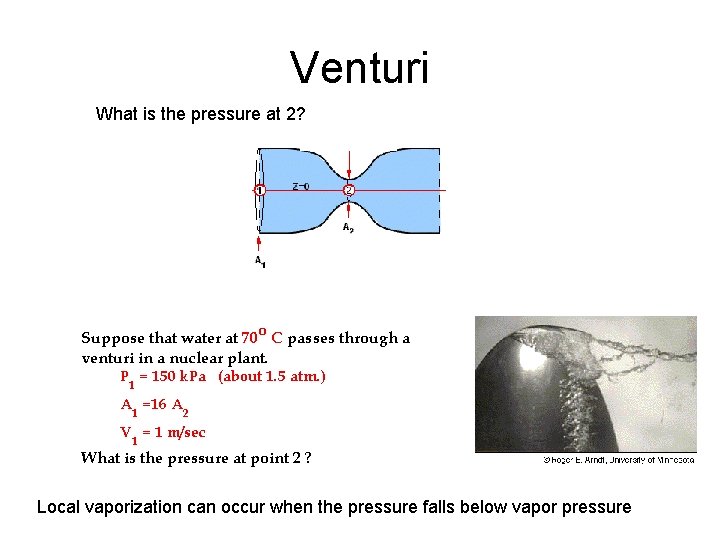
Venturi What is the pressure at 2? Local vaporization can occur when the pressure falls below vapor pressure