Chapter 18 Synaptic plasticity From Cellular and Molecular



- Slides: 27

Chapter 18 Synaptic plasticity From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved.

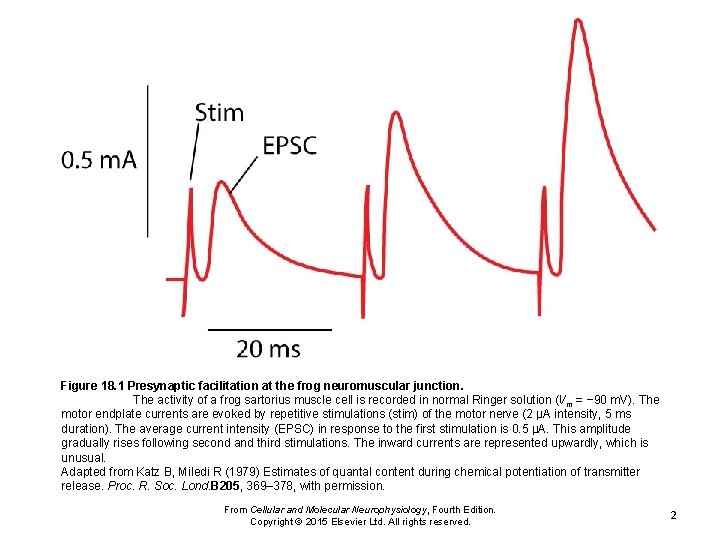
Figure 18. 1 Presynaptic facilitation at the frog neuromuscular junction. The activity of a frog sartorius muscle cell is recorded in normal Ringer solution (Vm = − 90 m. V). The motor endplate currents are evoked by repetitive stimulations (stim) of the motor nerve (2 μA intensity, 5 ms duration). The average current intensity (EPSC) in response to the first stimulation is 0. 5 μA. This amplitude gradually rises following second and third stimulations. The inward currents are represented upwardly, which is unusual. Adapted from Katz B, Miledi R (1979) Estimates of quantal content during chemical potentiation of transmitter release. Proc. R. Soc. Lond. B 205, 369– 378, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 2

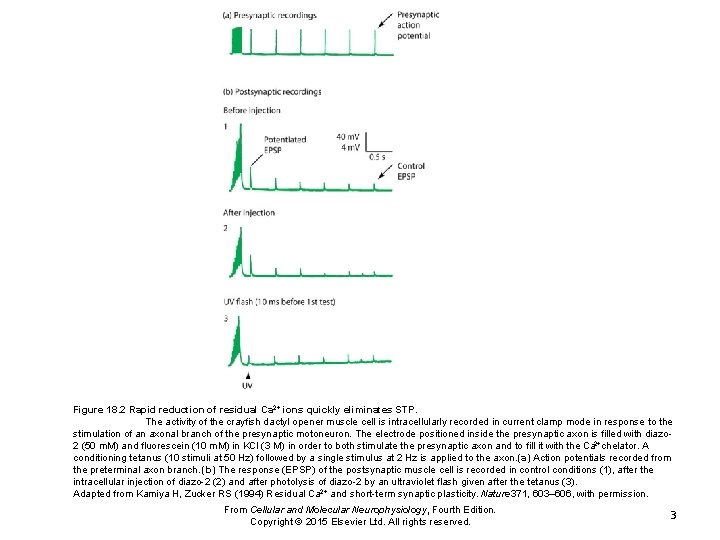
Figure 18. 2 Rapid reduction of residual Ca 2+ ions quickly eliminates STP. The activity of the crayfish dactyl opener muscle cell is intracellularly recorded in current clamp mode in response to the stimulation of an axonal branch of the presynaptic motoneuron. The electrode positioned inside the presynaptic axon is filled with diazo 2 (50 m. M) and fluorescein (10 m. M) in KCl (3 M) in order to both stimulate the presynaptic axon and to fill it with the Ca 2+chelator. A conditioning tetanus (10 stimuli at 50 Hz) followed by a single stimulus at 2 Hz is applied to the axon. (a) Action potentials recorded from the preterminal axon branch. (b) The response (EPSP) of the postsynaptic muscle cell is recorded in control conditions (1), after the intracellular injection of diazo-2 (2) and after photolysis of diazo-2 by an ultraviolet flash given after the tetanus (3). Adapted from Kamiya H, Zucker RS (1994) Residual Ca 2+ and short-term synaptic plasticity. Nature 371, 603– 606, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 3

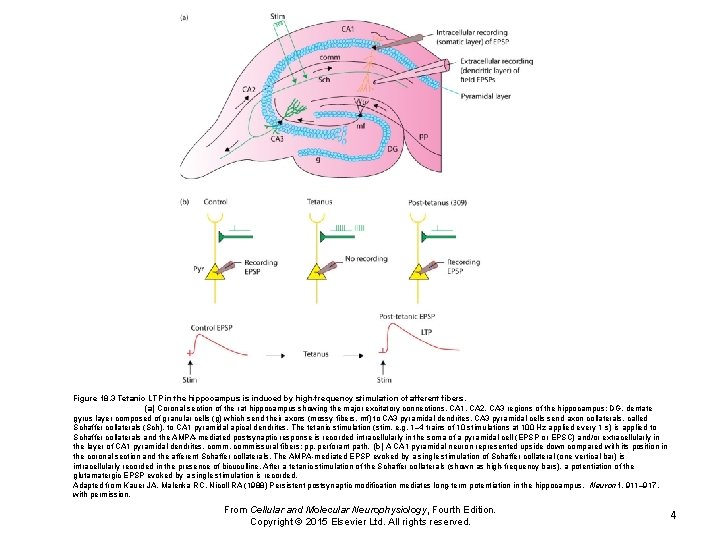
Figure 18. 3 Tetanic LTP in the hippocampus is induced by high-frequency stimulation of afferent fibers. (a) Coronal section of the rat hippocampus showing the major excitatory connections. CA 1, CA 2, CA 3 regions of the hippocampus; DG, dentate gyrus layer composed of granular cells (g) which send their axons (mossy fibers, mf) to CA 3 pyramidal dendrites. CA 3 pyramidal cells send axon collaterals, called Schaffer collaterals (Sch), to CA 1 pyramidal apical dendrites. The tetanic stimulation (stim, e. g. 1– 4 trains of 10 stimulations at 100 Hz applied every 1 s) is applied to Schaffer collaterals and the AMPA-mediated postsynaptic response is recorded intracellularly in the soma of a pyramidal cell (EPSP or EPSC) and/or extracellularly in the layer of CA 1 pyramidal dendrites. comm, commissural fibers; pp, perforant path. (b) A CA 1 pyramidal neuron represented upside down compared with its position in the coronal section and the afferent Schaffer collaterals. The AMPA-mediated EPSP evoked by a single stimulation of Schaffer collateral (one vertical bar) is intracellularly recorded in the presence of bicuculline. After a tetanic stimulation of the Schaffer collaterals (shown as high-frequency bars), a potentiation of the glutamatergic EPSP evoked by a single stimulation is recorded. Adapted from Kauer JA, Malenka RC, Nicoll RA (1988) Persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron 1, 911– 917, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 4

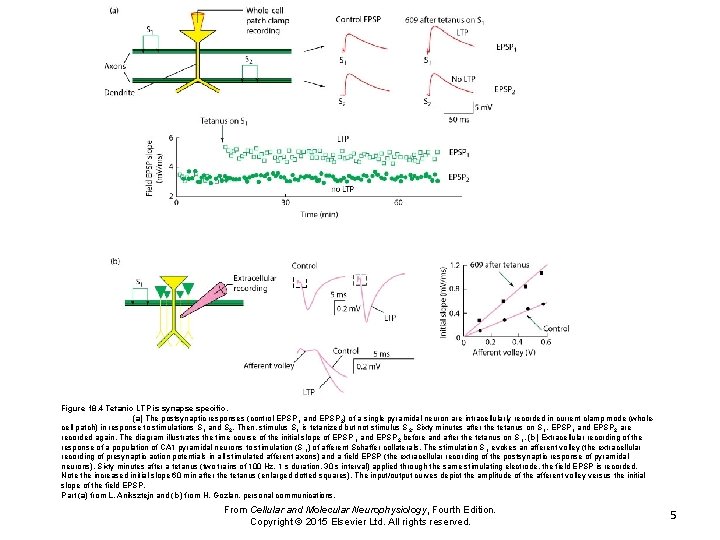
Figure 18. 4 Tetanic LTP is synapse specific. (a) The postsynaptic responses (control EPSP 1 and EPSP 2) of a single pyramidal neuron are intracellularly recorded in current clamp mode (wholecell patch) in response to stimulations S 1 and S 2. Then, stimulus S 1 is tetanized but not stimulus S 2. Sixty minutes after the tetanus on S 1, EPSP 1 and EPSP 2 are recorded again. The diagram illustrates the time course of the initial slope of EPSP 1 and EPSP 2 before and after the tetanus on S 1. (b) Extracellular recording of the response of a population of CA 1 pyramidal neurons to stimulation (S 1) of afferent Schaffer collaterals. The stimulation S 1 evokes an afferent volley (the extracellular recording of presynaptic action potentials in all stimulated afferent axons) and a field EPSP (the extracellular recording of the postsynaptic response of pyramidal neurons). Sixty minutes after a tetanus (two trains of 100 Hz, 1 s duration, 30 s interval) applied through the same stimulating electrode, the field EPSP is recorded. Note the increased initial slope 60 min after the tetanus (enlarged dotted squares). The input/output curves depict the amplitude of the afferent volley versus the initial slope of the field EPSP. Part (a) from L. Aniksztejn and (b) from H. Gozlan, personal communications. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 5

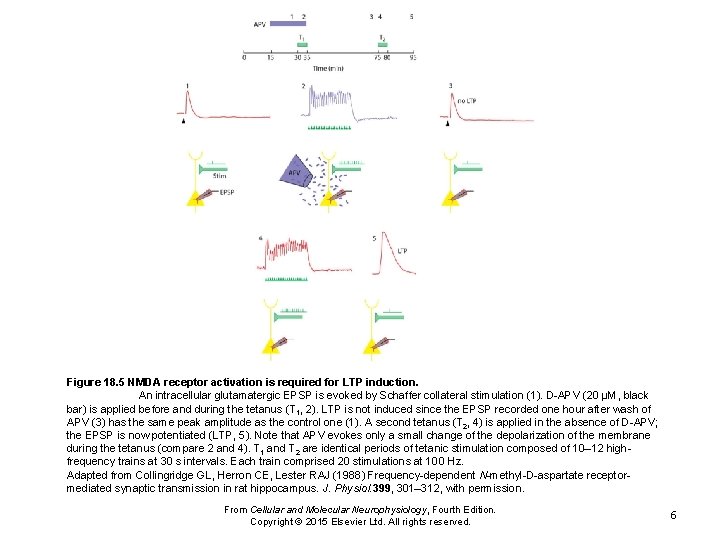
Figure 18. 5 NMDA receptor activation is required for LTP induction. An intracellular glutamatergic EPSP is evoked by Schaffer collateral stimulation (1). D-APV (20 μM, black bar) is applied before and during the tetanus (T 1, 2). LTP is not induced since the EPSP recorded one hour after wash of APV (3) has the same peak amplitude as the control one (1). A second tetanus (T 2, 4) is applied in the absence of D-APV; the EPSP is now potentiated (LTP, 5). Note that APV evokes only a small change of the depolarization of the membrane during the tetanus (compare 2 and 4). T 1 and T 2 are identical periods of tetanic stimulation composed of 10– 12 highfrequency trains at 30 s intervals. Each train comprised 20 stimulations at 100 Hz. Adapted from Collingridge GL, Herron CE, Lester RAJ (1988) Frequency-dependent N-methyl-D-aspartate receptormediated synaptic transmission in rat hippocampus. J. Physiol. 399, 301– 312, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 6

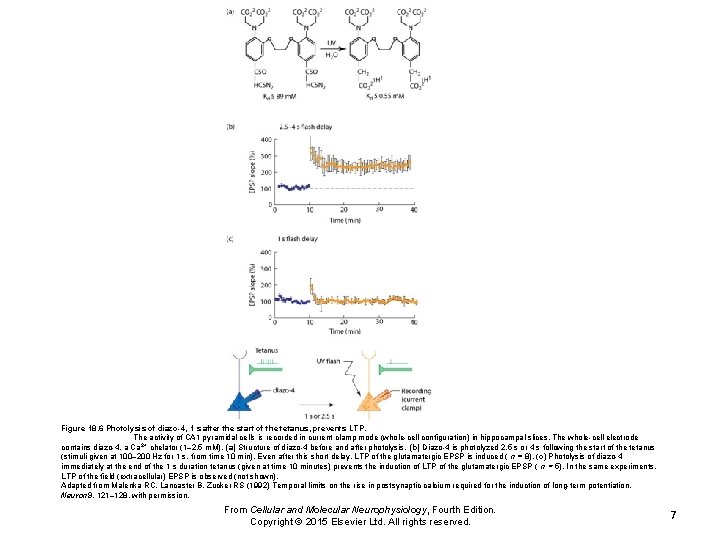
Figure 18. 6 Photolysis of diazo-4, 1 s after the start of the tetanus, prevents LTP. The activity of CA 1 pyramidal cells is recorded in current clamp mode (whole-cell configuration) in hippocampal slices. The whole-cell electrode contains diazo-4, a Ca 2+ chelator (1– 2. 5 m. M). (a) Structure of diazo-4 before and after photolysis. (b) Diazo-4 is photolyzed 2. 5 s or 4 s following the start of the tetanus (stimuli given at 100– 200 Hz for 1 s, from time 10 min). Even after this short delay, LTP of the glutamatergic EPSP is induced ( n = 8). (c) Photolysis of diazo-4 immediately at the end of the 1 s duration tetanus (given at time 10 minutes) prevents the induction of LTP of the glutamatergic EPSP ( n = 5). In the same experiments, LTP of the field (extracellular) EPSP is observed (not shown). Adapted from Malenka RC, Lancaster B, Zucker RS (1992) Temporal limits on the rise in postsynaptic calcium required for the induction of long-term potentiation. Neuron 9, 121– 128, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 7

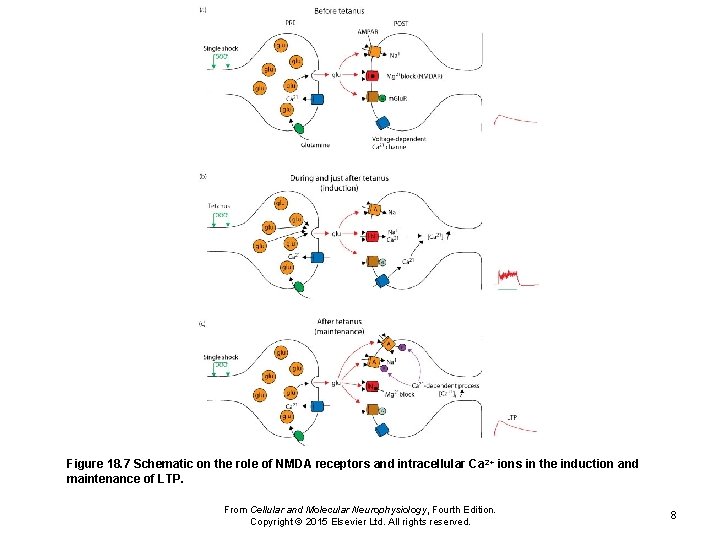
Figure 18. 7 Schematic on the role of NMDA receptors and intracellular Ca 2+ ions in the induction and maintenance of LTP. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 8

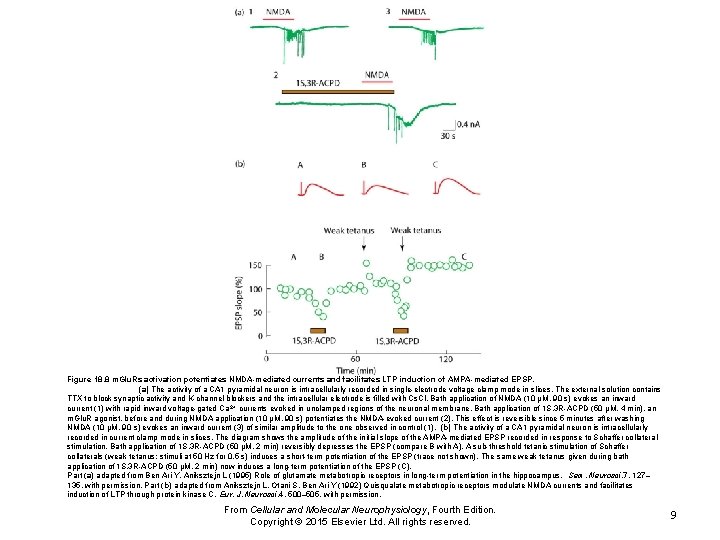
Figure 18. 8 m. Glu. Rs activation potentiates NMDA-mediated currents and facilitates LTP induction of AMPA-mediated EPSP. (a) The activity of a CA 1 pyramidal neuron is intracellularly recorded in single-electrode voltage clamp mode in slices. The external solution contains TTX to block synaptic activity and K-channel blockers and the intracellular electrode is filled with Cs. Cl. Bath application of NMDA (10 μM, 90 s) evokes an inward current (1) with rapid inward voltage-gated Ca 2+ currents evoked in unclamped regions of the neuronal membrane. Bath application of 1 S, 3 R-ACPD (50 μM, 4 min), an m. Glu. R agonist, before and during NMDA application (10 μM, 90 s) potentiates the NMDA-evoked current (2). This effect is reversible since 5 minutes after washing NMDA (10 μM, 90 s) evokes an inward current (3) of similar amplitude to the one observed in control (1). (b) The activity of a CA 1 pyramidal neuron is intracellularly recorded in current clamp mode in slices. The diagram shows the amplitude of the initial slope of the AMPA-mediated EPSP recorded in response to Schaffer collateral stimulation. Bath application of 1 S, 3 R-ACPD (50 μM, 2 min) reversibly depresses the EPSP (compare B with A). A sub-threshold tetanic stimulation of Schaffer collaterals (weak tetanus: stimuli at 50 Hz for 0. 5 s) induces a short-term potentiation of the EPSP (trace not shown). The same weak tetanus given during bath application of 1 S, 3 R-ACPD (50 μM, 2 min) now induces a long-term potentiation of the EPSP (C). Part (a) adapted from Ben Ari Y, Aniksztejn L (1995) Role of glutamate metabotropic receptors in long-term potentiation in the hippocampus. Sem. Neurosci. 7, 127– 135, with permission. Part (b) adapted from Aniksztejn L, Otani S, Ben Ari Y (1992) Quisqualate metabotropic receptors modulate NMDA currents and facilitates induction of LTP through protein kinase C. Eur. J. Neurosci. 4, 500– 505, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 9

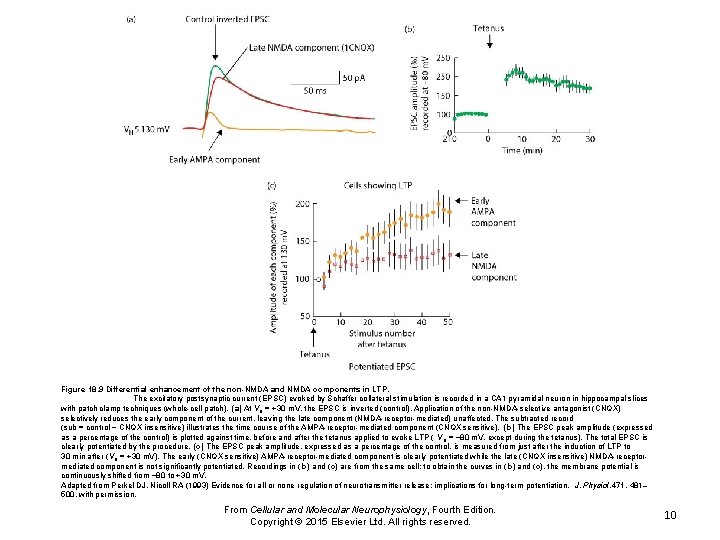
Figure 18. 9 Differential enhancement of the non-NMDA and NMDA components in LTP. The excitatory postsynaptic current (EPSC) evoked by Schaffer collateral stimulation is recorded in a CA 1 pyramidal neuron in hippocampal slices with patch clamp techniques (whole-cell patch). (a) At VH = +30 m. V, the EPSC is inverted (control). Application of the non-NMDA-selective antagonist (CNQX) selectively reduces the early component of the current, leaving the late component (NMDA-receptor-mediated) unaffected. The subtracted record (sub = control − CNQX insensitive) illustrates the time course of the AMPA-receptor-mediated component (CNQX sensitive). (b) The EPSC peak amplitude (expressed as a percentage of the control) is plotted against time, before and after the tetanus applied to evoke LTP ( VH = − 80 m. V, except during the tetanus). The total EPSC is clearly potentiated by the procedure. (c) The EPSC peak amplitude, expressed as a percentage of the control, is measured from just after the induction of LTP to 30 min after (VH = +30 m. V). The early (CNQX sensitive) AMPA-receptor-mediated component is clearly potentiated while the late (CNQX insensitive) NMDA-receptormediated component is not significantly potentiated. Recordings in ( b) and (c) are from the same cell; to obtain the curves in ( b) and (c), the membrane potential is continuously shifted from − 80 to +30 m. V. Adapted from Perkel DJ, Nicoll RA (1993) Evidence for all or none regulation of neurotransmitter release: implications for long-term potentiation. J. Physiol. 471, 481– 500, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 10

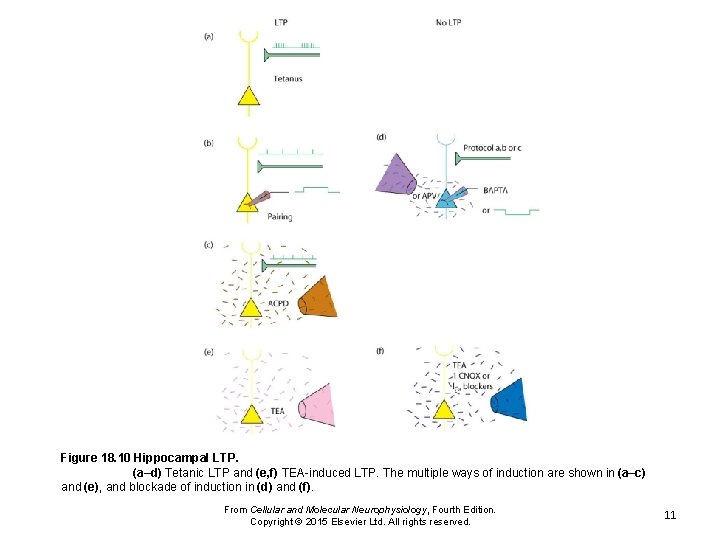
Figure 18. 10 Hippocampal LTP. (a–d) Tetanic LTP and (e, f) TEA-induced LTP. The multiple ways of induction are shown in (a–c) and (e), and blockade of induction in (d) and (f). From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 11

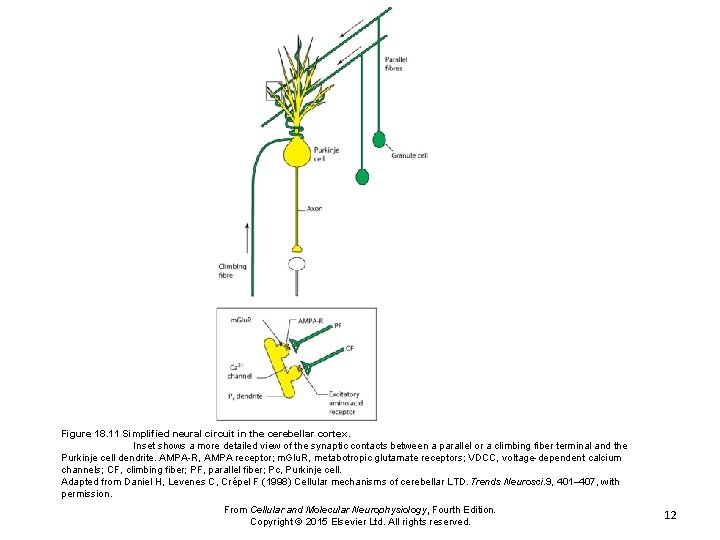
Figure 18. 11 Simplified neural circuit in the cerebellar cortex. Inset shows a more detailed view of the synaptic contacts between a parallel or a climbing fiber terminal and the Purkinje cell dendrite. AMPA-R, AMPA receptor; m. Glu. R, metabotropic glutamate receptors; VDCC, voltage-dependent calcium channels; CF, climbing fiber; PF, parallel fiber; Pc, Purkinje cell. Adapted from Daniel H, Levenes C, Crépel F (1998) Cellular mechanisms of cerebellar LTD. Trends Neurosci. 9, 401– 407, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 12

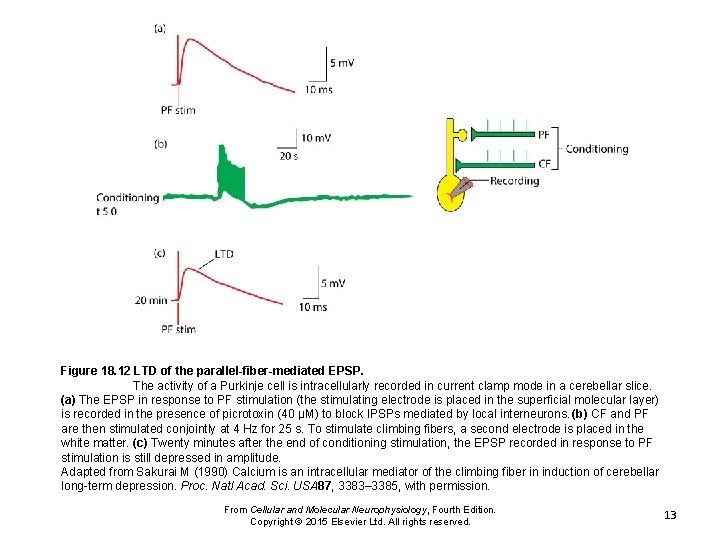
Figure 18. 12 LTD of the parallel-fiber-mediated EPSP. The activity of a Purkinje cell is intracellularly recorded in current clamp mode in a cerebellar slice. (a) The EPSP in response to PF stimulation (the stimulating electrode is placed in the superficial molecular layer) is recorded in the presence of picrotoxin (40 μM) to block IPSPs mediated by local interneurons. (b) CF and PF are then stimulated conjointly at 4 Hz for 25 s. To stimulate climbing fibers, a second electrode is placed in the white matter. (c) Twenty minutes after the end of conditioning stimulation, the EPSP recorded in response to PF stimulation is still depressed in amplitude. Adapted from Sakurai M (1990) Calcium is an intracellular mediator of the climbing fiber in induction of cerebellar long-term depression. Proc. Natl Acad. Sci. USA 87, 3383– 3385, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 13

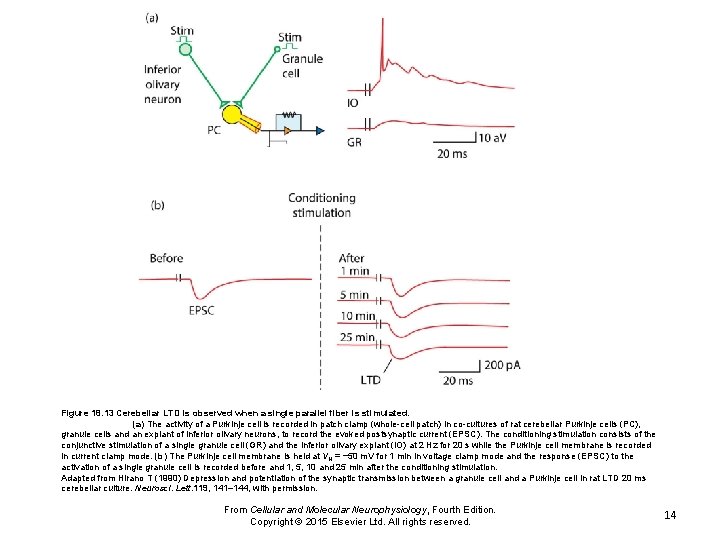
Figure 18. 13 Cerebellar LTD is observed when a single parallel fiber is stimulated. (a) The activity of a Purkinje cell is recorded in patch clamp (whole-cell patch) in co-cultures of rat cerebellar Purkinje cells (PC), granule cells and an explant of inferior olivary neurons, to record the evoked postsynaptic current (EPSC). The conditioning stimulation consists of the conjunctive stimulation of a single granule cell (GR) and the inferior olivary explant (IO) at 2 Hz for 20 s while the Purkinje cell membrane is recorded in current clamp mode. (b) The Purkinje cell membrane is held at VH = − 50 m. V for 1 min in voltage clamp mode and the response (EPSC) to the activation of a single granule cell is recorded before and 1, 5, 10 and 25 min after the conditioning stimulation. Adapted from Hirano T (1990) Depression and potentiation of the synaptic transmission between a granule cell and a Purkinje cell in rat LTD 20 ms cerebellar culture. Neurosci. Lett. 119, 141– 144, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 14

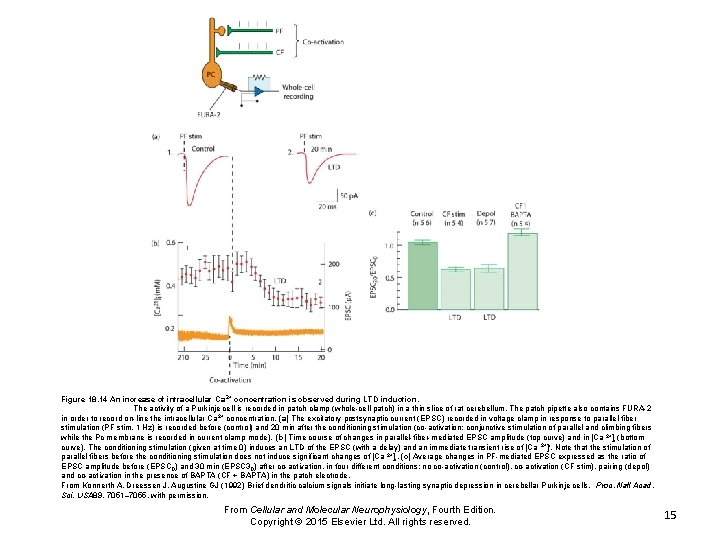
Figure 18. 14 An increase of intracellular Ca 2+ concentration is observed during LTD induction. The activity of a Purkinje cell is recorded in patch clamp (whole-cell patch) in a thin slice of rat cerebellum. The patch pipette also contains FURA-2 in order to record on-line the intracellular Ca 2+ concentration. (a) The excitatory postsynaptic current (EPSC) recorded in voltage clamp in response to parallel fiber stimulation (PF stim, 1 Hz) is recorded before (control) and 20 min after the conditioning stimulation (co-activation: conjunctive stimulation of parallel and climbing fibers while the Pc membrane is recorded in current clamp mode). (b) Time course of changes in parallel-fiber-mediated EPSC amplitude (top curve) and in [Ca 2+]i (bottom curve). The conditioning stimulation (given at time 0) induces an LTD of the EPSC (with a delay) and an immediate transient rise of [Ca 2+]i. Note that the stimulation of parallel fibers before the conditioning stimulation does not induce significant changes of [Ca 2+]i. (c) Average changes in PF-mediated EPSC expressed as the ratio of EPSC amplitude before (EPSC 0) and 30 min (EPSC 3 0) after co-activation, in four different conditions: no co-activation (control), co-activation (CF stim), pairing (depol) and co-activation in the presence of BAPTA (CF + BAPTA) in the patch electrode. From Konnerth A, Dreessen J, Augustine GJ (1992) Brief dendritic calcium signals initiate long-lasting synaptic depression in cerebellar Purkinje cells. Proc. Natl Acad. Sci. USA 89, 7051– 7055, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 15

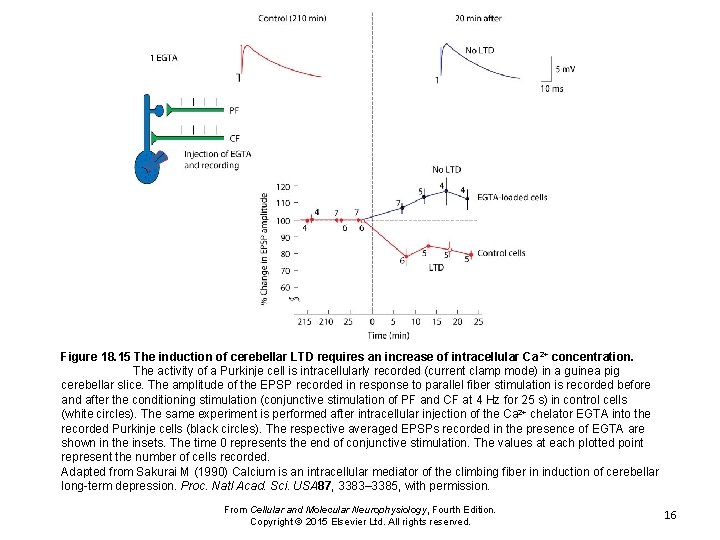
Figure 18. 15 The induction of cerebellar LTD requires an increase of intracellular Ca 2+ concentration. The activity of a Purkinje cell is intracellularly recorded (current clamp mode) in a guinea pig cerebellar slice. The amplitude of the EPSP recorded in response to parallel fiber stimulation is recorded before and after the conditioning stimulation (conjunctive stimulation of PF and CF at 4 Hz for 25 s) in control cells (white circles). The same experiment is performed after intracellular injection of the Ca 2+ chelator EGTA into the recorded Purkinje cells (black circles). The respective averaged EPSPs recorded in the presence of EGTA are shown in the insets. The time 0 represents the end of conjunctive stimulation. The values at each plotted point represent the number of cells recorded. Adapted from Sakurai M (1990) Calcium is an intracellular mediator of the climbing fiber in induction of cerebellar long-term depression. Proc. Natl Acad. Sci. USA 87, 3383– 3385, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 16

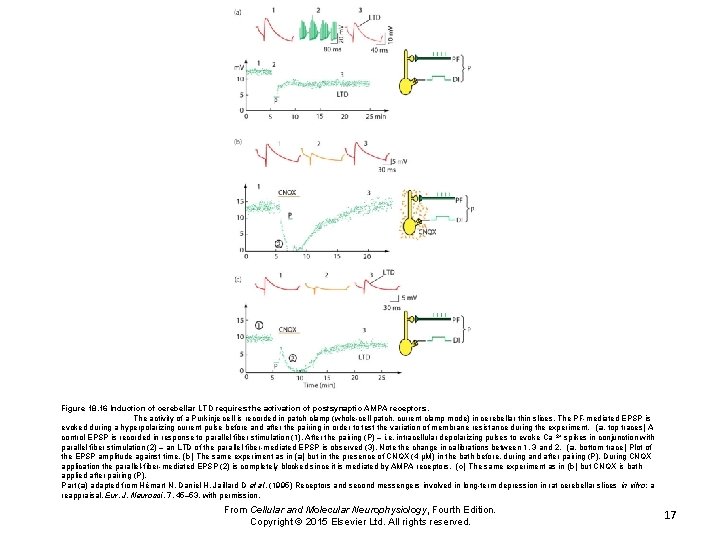
Figure 18. 16 Induction of cerebellar LTD requires the activation of postsynaptic AMPA receptors. The activity of a Purkinje cell is recorded in patch clamp (whole-cell patch, current clamp mode) in cerebellar thin slices. The PF-mediated EPSP is evoked during a hyperpolarizing current pulse before and after the pairing in order to test the variation of membrane resistance during the experiment. (a, top traces) A control EPSP is recorded in response to parallel fiber stimulation (1). After the pairing (P) – i. e. intracellular depolarizing pulses to evoke Ca 2+ spikes in conjunction with parallel fiber stimulation (2) – an LTD of the parallel-fiber-mediated EPSP is observed (3). Note the change in calibrations between 1, 3 and 2. (a, bottom trace) Plot of the EPSP amplitude against time. (b) The same experiment as in (a) but in the presence of CNQX (4 μM) in the bath before, during and after pairing (P). During CNQX application the parallel-fiber-mediated EPSP (2) is completely blocked since it is mediated by AMPA receptors. (c) The same experiment as in (b) but CNQX is bath applied after pairing (P). Part (a) adapted from Hémart N, Daniel H, Jaillard D et al. (1995) Receptors and second messengers involved in long-term depression in rat cerebellar slices in vitro: a reappraisal. Eur. J. Neurosci. 7, 45– 53, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 17

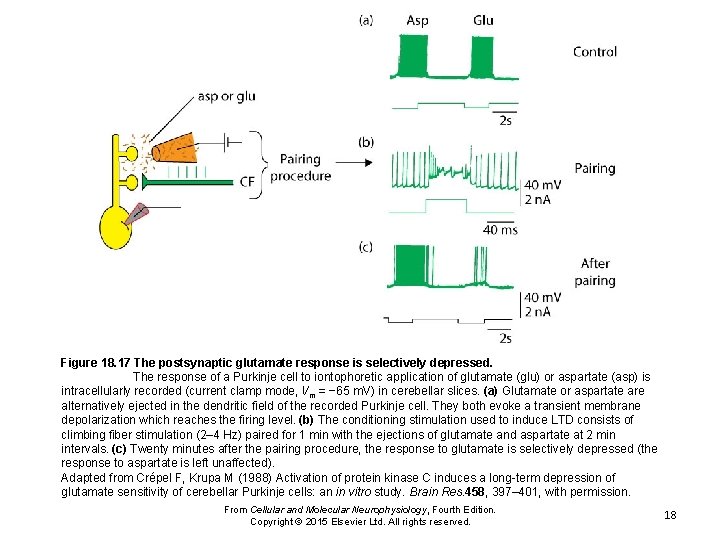
Figure 18. 17 The postsynaptic glutamate response is selectively depressed. The response of a Purkinje cell to iontophoretic application of glutamate (glu) or aspartate (asp) is intracellularly recorded (current clamp mode, Vm = − 65 m. V) in cerebellar slices. (a) Glutamate or aspartate are alternatively ejected in the dendritic field of the recorded Purkinje cell. They both evoke a transient membrane depolarization which reaches the firing level. (b) The conditioning stimulation used to induce LTD consists of climbing fiber stimulation (2– 4 Hz) paired for 1 min with the ejections of glutamate and aspartate at 2 min intervals. (c) Twenty minutes after the pairing procedure, the response to glutamate is selectively depressed (the response to aspartate is left unaffected). Adapted from Crépel F, Krupa M (1988) Activation of protein kinase C induces a long-term depression of glutamate sensitivity of cerebellar Purkinje cells: an in vitro study. Brain Res. 458, 397– 401, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 18

Figure 18. 18 Protein kinase C inhibition prevents LTD induction. The activity of a Purkinje cell is recorded in patch clamp (whole-cell patch) in the presence of the selective PKC inhibitor, PKC 19 -36 (circles) or a non-inhibitory control peptide (triangle) or the intracellular solution only (square) in the patch pipette. The control EPSC evoked by parallel fiber stimulation is recorded for 10 min and the conditioning stimulation is applied att = 0. It consists of the conjunctive application of glutamate and intracellular depolarization. LTD of the EPSC is not observed in cells dialyzed with PKC 19 -36. Scale bars: 100 p. A, 2 s. Adapted from Linden DJ, Connor JA (1991) Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science 254, 1656– 1659, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 19

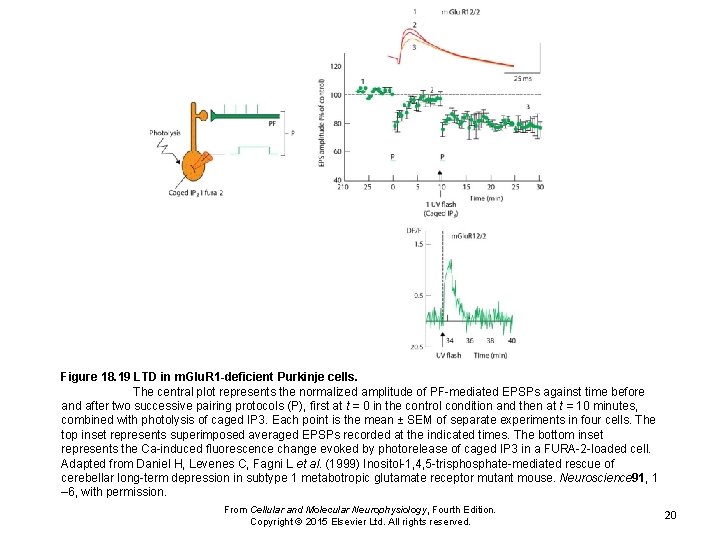
Figure 18. 19 LTD in m. Glu. R 1 -deficient Purkinje cells. The central plot represents the normalized amplitude of PF-mediated EPSPs against time before and after two successive pairing protocols (P), first at t = 0 in the control condition and then at t = 10 minutes, combined with photolysis of caged IP 3. Each point is the mean ± SEM of separate experiments in four cells. The top inset represents superimposed averaged EPSPs recorded at the indicated times. The bottom inset represents the Ca-induced fluorescence change evoked by photorelease of caged IP 3 in a FURA-2 -loaded cell. Adapted from Daniel H, Levenes C, Fagni L et al. (1999) Inositol-1, 4, 5 -trisphosphate-mediated rescue of cerebellar long-term depression in subtype 1 metabotropic glutamate receptor mutant mouse. Neuroscience 91, 1 – 6, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 20

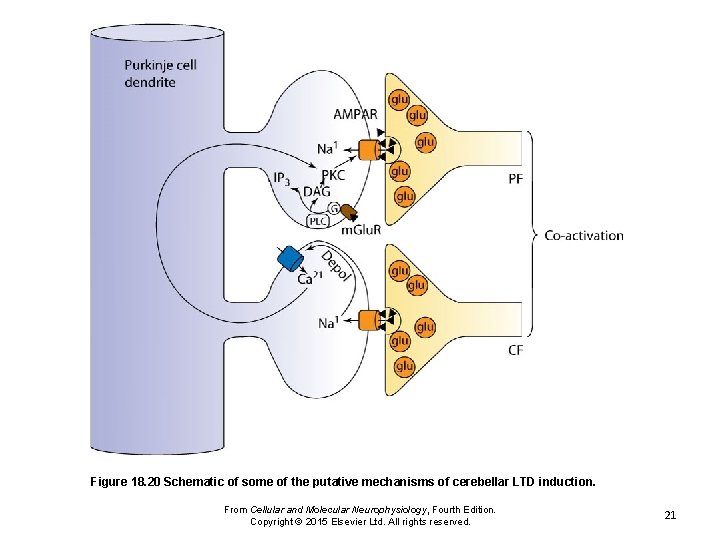
Figure 18. 20 Schematic of some of the putative mechanisms of cerebellar LTD induction. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 21

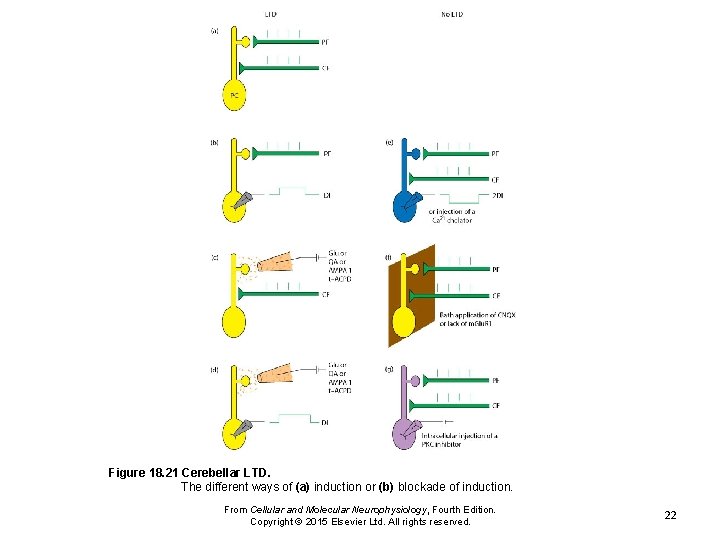
Figure 18. 21 Cerebellar LTD. The different ways of (a) induction or (b) blockade of induction. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 22

Figure 18. 22 Spike-timing-dependent plasticity (STDP) at hippocampal synapses. (a) Two neurons connected by a synapse are recorded intracellularly. An action potential in the presynaptic neuron (blue) elicits an excitatory postsynaptic potential in the postsynaptic cell (red). (b) Long-term synaptic potentiation induced by positive correlation. Left, induction protocol (pairing indicated by horizontal bar in graph) consisting in single presynaptic action potential elicited 15 ms before a single postsynaptic action potential (repeated 50 times at 0. 3 Hz). Right, time-course of the potentiation. Averaged traces from the labeled time points are illustrated above the graph. (c) Long-term synaptic depression induced by negative correlation. Left, induction protocol repeated 100 times at 0. 3 Hz. Note that here, the postsynaptic spike precedes the presynaptic action potential (70 ms). Right, time-course of the depression. Averaged traces from the labeled time points are illustrated above the graph. Adapted from Debanne et al. (1998) J. Physiol. (d) STDP curve. The percentage of synaptic change is expressed as a function of the spike timing (the presynaptic spike is taken as the time reference). Synaptic potentiation is obtained for positive timing and depression for negative timing. Parts (b) and (c) adapted from Debanne, D. , Gähwiler, B. H. , Thompson, S. M. , 1998. Longterm synaptic plasticity between pairs of CA 3 pyramidal cells in hippocampal slice cultures. J. Physiol. 507, 237– 247, with permission. Part (d) adapted from Bi and Poo (1998) Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 18, 10464– 10472. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 23

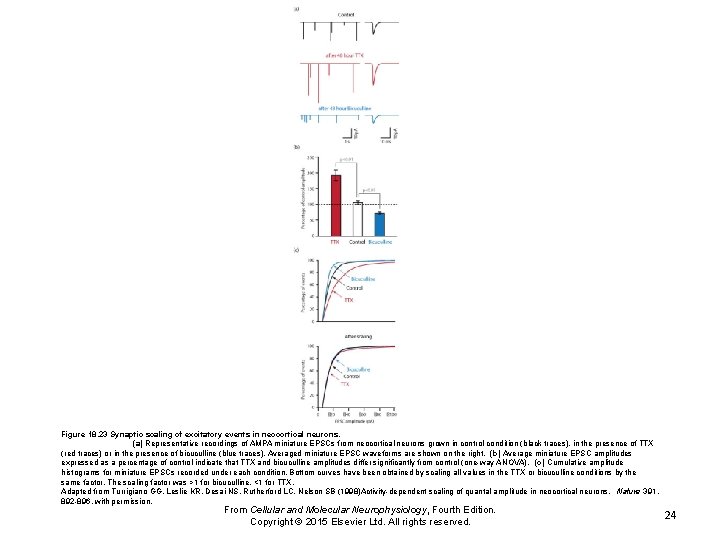
Figure 18. 23 Synaptic scaling of excitatory events in neocortical neurons. (a) Representative recordings of AMPA miniature EPSCs from neocortical neurons grown in control condition (black traces), in the presence of TTX (red traces) or in the presence of bicuculline (blue traces). Averaged miniature EPSC waveforms are shown on the right. (b) Average miniature EPSC amplitudes expressed as a percentage of control indicate that TTX and bicuculline amplitudes differ significantly from control (one-way ANOVA). (c) Cumulative amplitude histograms for miniature EPSCs recorded under each condition. Bottom curves have been obtained by scaling all values in the TTX or bicuculline conditions by the same factor. The scaling factor was >1 for bicuculline, <1 for TTX. Adapted from Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB (1998)Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892 -896, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 24

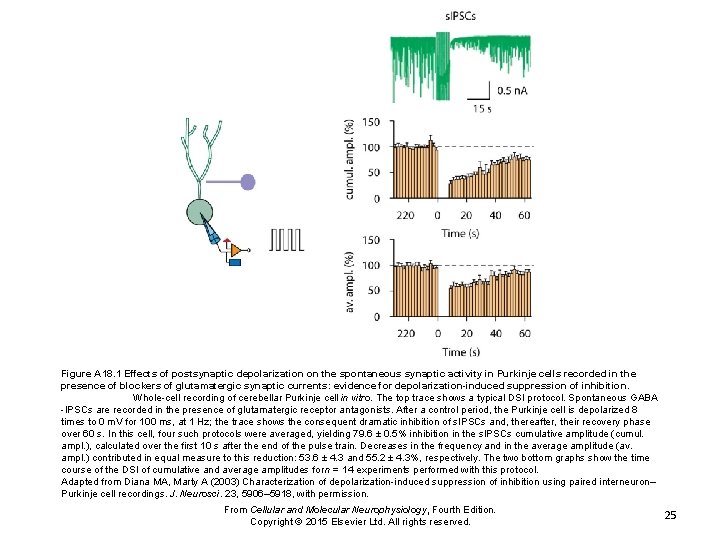
Figure A 18. 1 Effects of postsynaptic depolarization on the spontaneous synaptic activity in Purkinje cells recorded in the presence of blockers of glutamatergic synaptic currents: evidence for depolarization-induced suppression of inhibition. Whole-cell recording of cerebellar Purkinje cell in vitro. The top trace shows a typical DSI protocol. Spontaneous GABA -IPSCs are recorded in the presence of glutamatergic receptor antagonists. After a control period, the Purkinje cell is depolarized 8 times to 0 m. V for 100 ms, at 1 Hz; the trace shows the consequent dramatic inhibition of s. IPSCs and, thereafter, their recovery phase over 60 s. In this cell, four such protocols were averaged, yielding 79. 6 ± 0. 5% inhibition in the s. IPSCs cumulative amplitude (cumul. ampl. ), calculated over the first 10 s after the end of the pulse train. Decreases in the frequency and in the average amplitude (av. ampl. ) contributed in equal measure to this reduction: 53. 6 ± 4. 3 and 55. 2 ± 4. 3%, respectively. The two bottom graphs show the time course of the DSI of cumulative and average amplitudes for n = 14 experiments performed with this protocol. Adapted from Diana MA, Marty A (2003) Characterization of depolarization-induced suppression of inhibition using paired interneuron– Purkinje cell recordings. J. Neurosci. 23, 5906– 5918, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 25

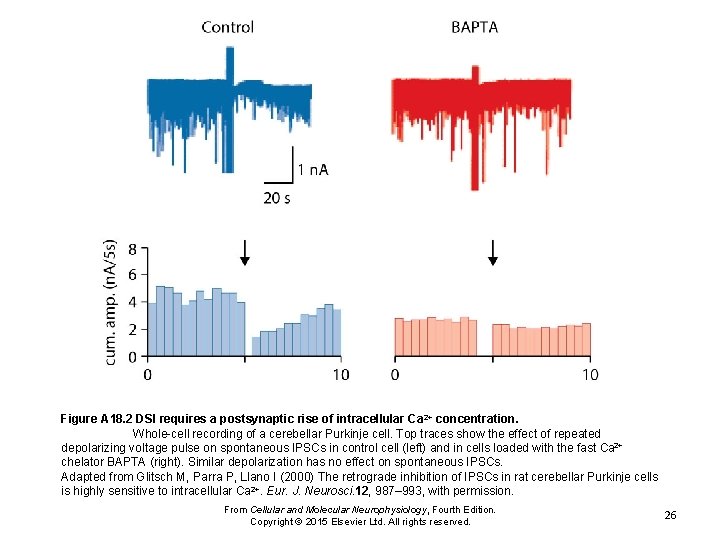
Figure A 18. 2 DSI requires a postsynaptic rise of intracellular Ca 2+ concentration. Whole-cell recording of a cerebellar Purkinje cell. Top traces show the effect of repeated depolarizing voltage pulse on spontaneous IPSCs in control cell (left) and in cells loaded with the fast Ca 2+ chelator BAPTA (right). Similar depolarization has no effect on spontaneous IPSCs. Adapted from Glitsch M, Parra P, Llano I (2000) The retrograde inhibition of IPSCs in rat cerebellar Purkinje cells is highly sensitive to intracellular Ca 2+. Eur. J. Neurosci. 12, 987– 993, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 26

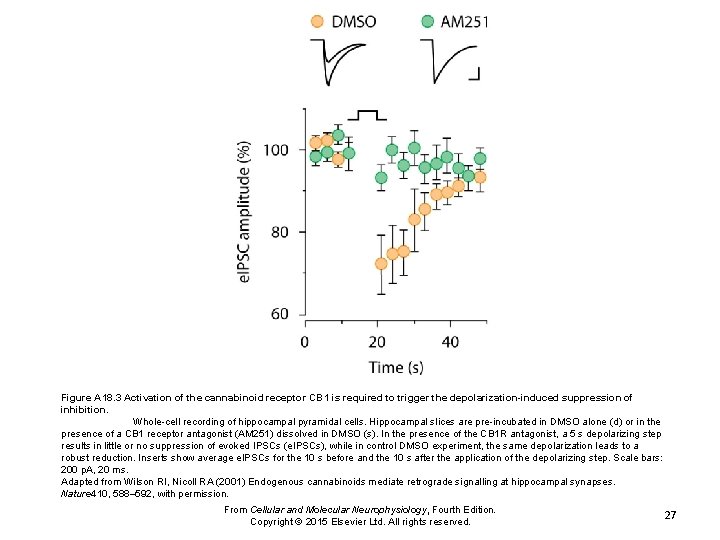
Figure A 18. 3 Activation of the cannabinoid receptor CB 1 is required to trigger the depolarization-induced suppression of inhibition. Whole-cell recording of hippocampal pyramidal cells. Hippocampal slices are pre-incubated in DMSO alone (d) or in the presence of a CB 1 receptor antagonist (AM 251) dissolved in DMSO (s). In the presence of the CB 1 R antagonist, a 5 s depolarizing step results in little or no suppression of evoked IPSCs (e. IPSCs), while in control DMSO experiment, the same depolarization leads to a robust reduction. Inserts show average e. IPSCs for the 10 s before and the 10 s after the application of the depolarizing step. Scale bars: 200 p. A, 20 ms. Adapted from Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588– 592, with permission. From Cellular and Molecular Neurophysiology, Fourth Edition. Copyright © 2015 Elsevier Ltd. All rights reserved. 27