Chapter 13 Stem Cells and Regenerative Medicine 2020

- Slides: 14

Chapter 13 Stem Cells and Regenerative Medicine © 2020 Elsevier Inc. All rights reserved.

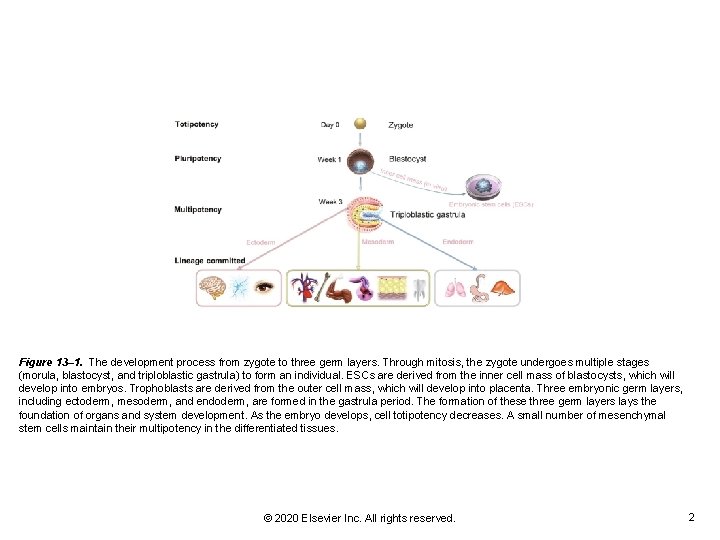
Figure 13– 1. The development process from zygote to three germ layers. Through mitosis, the zygote undergoes multiple stages (morula, blastocyst, and triploblastic gastrula) to form an individual. ESCs are derived from the inner cell mass of blastocysts, which will develop into embryos. Trophoblasts are derived from the outer cell mass, which will develop into placenta. Three embryonic germ layers, including ectoderm, mesoderm, and endoderm, are formed in the gastrula period. The formation of these three germ layers lays the foundation of organs and system development. As the embryo develops, cell totipotency decreases. A small number of mesenchymal stem cells maintain their multipotency in the differentiated tissues. © 2020 Elsevier Inc. All rights reserved. 2

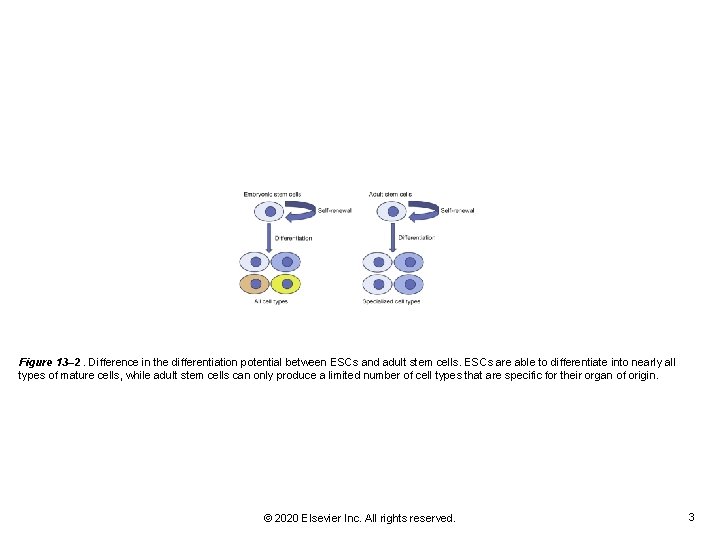
Figure 13– 2. Difference in the differentiation potential between ESCs and adult stem cells. ESCs are able to differentiate into nearly all types of mature cells, while adult stem cells can only produce a limited number of cell types that are specific for their organ of origin. © 2020 Elsevier Inc. All rights reserved. 3

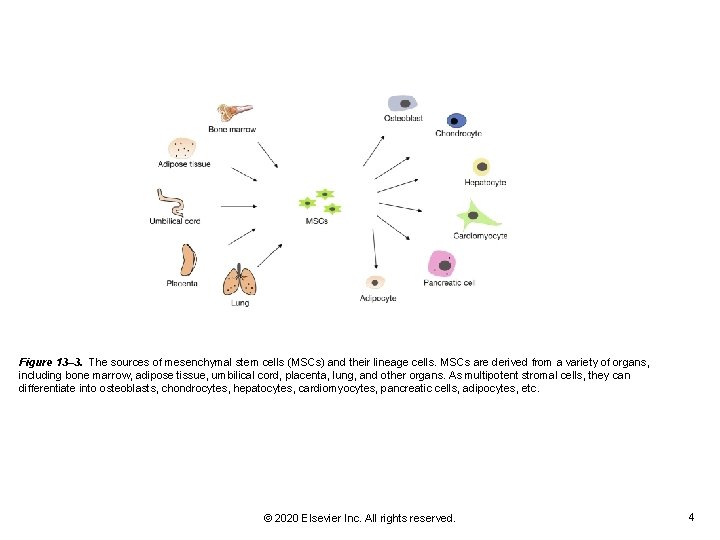
Figure 13– 3. The sources of mesenchymal stem cells (MSCs) and their lineage cells. MSCs are derived from a variety of organs, including bone marrow, adipose tissue, umbilical cord, placenta, lung, and other organs. As multipotent stromal cells, they can differentiate into osteoblasts, chondrocytes, hepatocytes, cardiomyocytes, pancreatic cells, adipocytes, etc. © 2020 Elsevier Inc. All rights reserved. 4

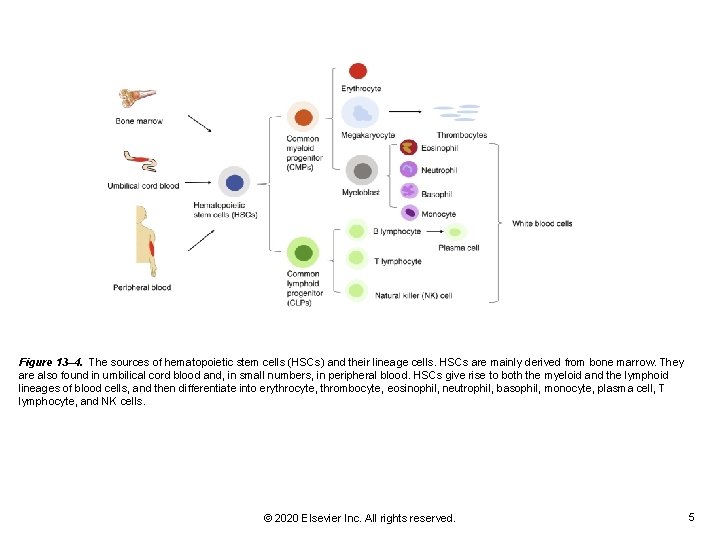
Figure 13– 4. The sources of hematopoietic stem cells (HSCs) and their lineage cells. HSCs are mainly derived from bone marrow. They are also found in umbilical cord blood and, in small numbers, in peripheral blood. HSCs give rise to both the myeloid and the lymphoid lineages of blood cells, and then differentiate into erythrocyte, thrombocyte, eosinophil, neutrophil, basophil, monocyte, plasma cell, T lymphocyte, and NK cells. © 2020 Elsevier Inc. All rights reserved. 5

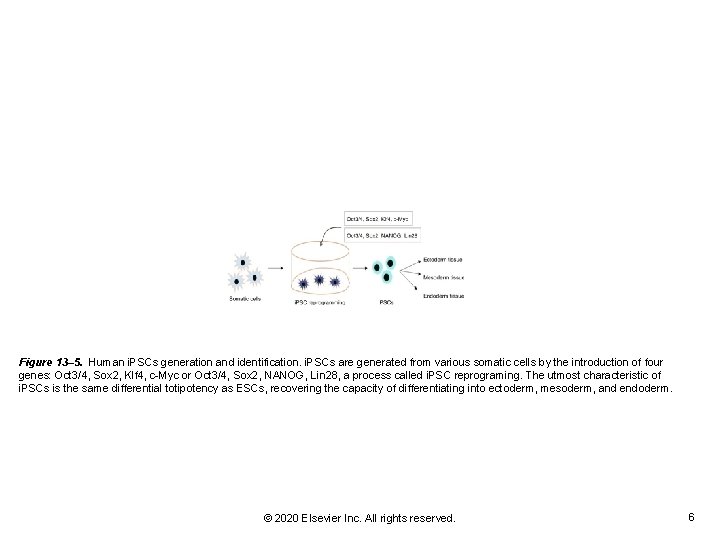
Figure 13– 5. Human i. PSCs generation and identification. i. PSCs are generated from various somatic cells by the introduction of four genes: Oct 3/4, Sox 2, Klf 4, c-Myc or Oct 3/4, Sox 2, NANOG, Lin 28, a process called i. PSC reprograming. The utmost characteristic of i. PSCs is the same differential totipotency as ESCs, recovering the capacity of differentiating into ectoderm, mesoderm, and endoderm. © 2020 Elsevier Inc. All rights reserved. 6

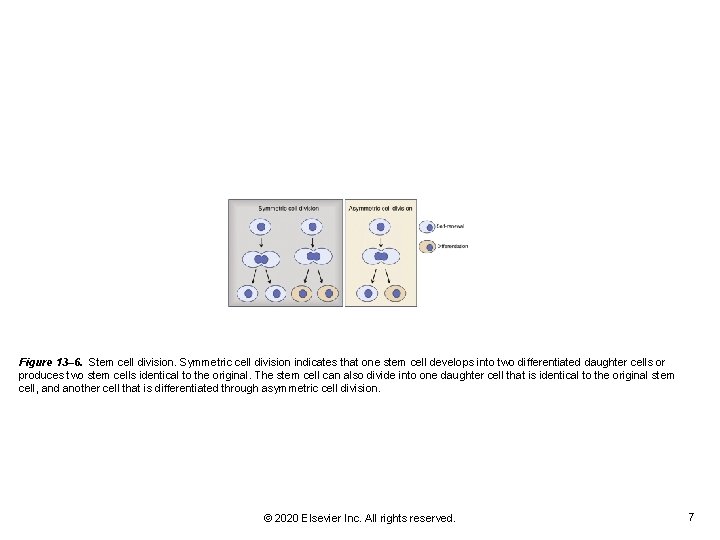
Figure 13– 6. Stem cell division. Symmetric cell division indicates that one stem cell develops into two differentiated daughter cells or produces two stem cells identical to the original. The stem cell can also divide into one daughter cell that is identical to the original stem cell, and another cell that is differentiated through asymmetric cell division. © 2020 Elsevier Inc. All rights reserved. 7

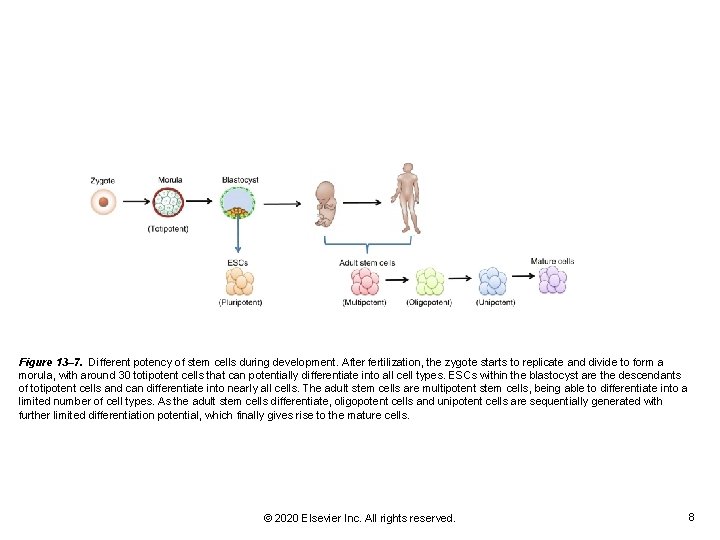
Figure 13– 7. Different potency of stem cells during development. After fertilization, the zygote starts to replicate and divide to form a morula, with around 30 totipotent cells that can potentially differentiate into all cell types. ESCs within the blastocyst are the descendants of totipotent cells and can differentiate into nearly all cells. The adult stem cells are multipotent stem cells, being able to differentiate into a limited number of cell types. As the adult stem cells differentiate, oligopotent cells and unipotent cells are sequentially generated with further limited differentiation potential, which finally gives rise to the mature cells. © 2020 Elsevier Inc. All rights reserved. 8

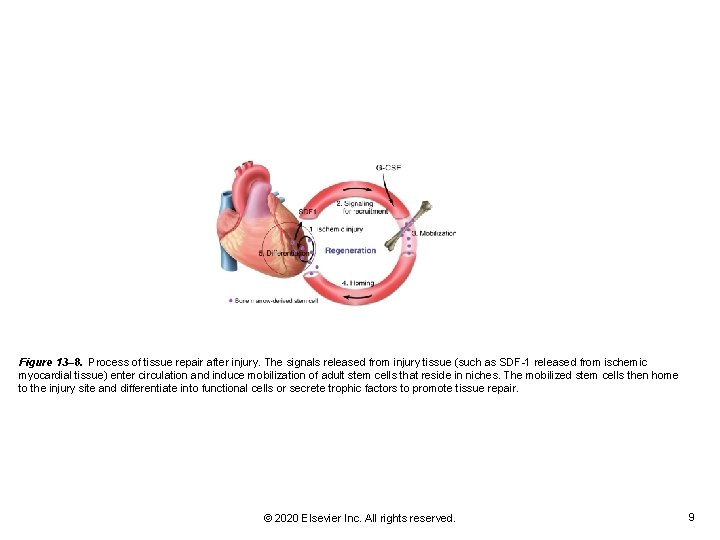
Figure 13– 8. Process of tissue repair after injury. The signals released from injury tissue (such as SDF-1 released from ischemic myocardial tissue) enter circulation and induce mobilization of adult stem cells that reside in niches. The mobilized stem cells then home to the injury site and differentiate into functional cells or secrete trophic factors to promote tissue repair. © 2020 Elsevier Inc. All rights reserved. 9

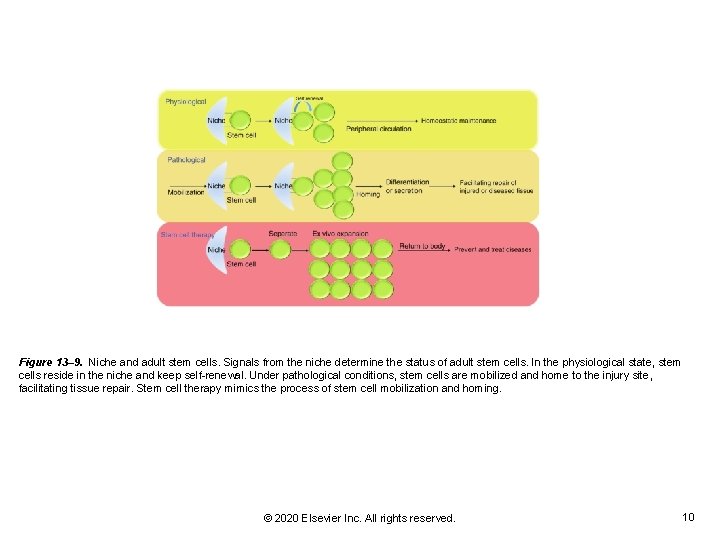
Figure 13– 9. Niche and adult stem cells. Signals from the niche determine the status of adult stem cells. In the physiological state, stem cells reside in the niche and keep self-renewal. Under pathological conditions, stem cells are mobilized and home to the injury site, facilitating tissue repair. Stem cell therapy mimics the process of stem cell mobilization and homing. © 2020 Elsevier Inc. All rights reserved. 10

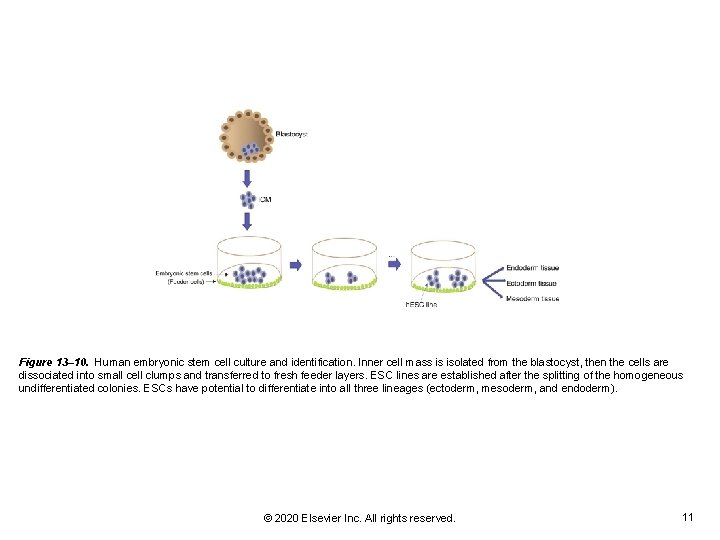
Figure 13– 10. Human embryonic stem cell culture and identification. Inner cell mass is isolated from the blastocyst, then the cells are dissociated into small cell clumps and transferred to fresh feeder layers. ESC lines are established after the splitting of the homogeneous undifferentiated colonies. ESCs have potential to differentiate into all three lineages (ectoderm, mesoderm, and endoderm). © 2020 Elsevier Inc. All rights reserved. 11

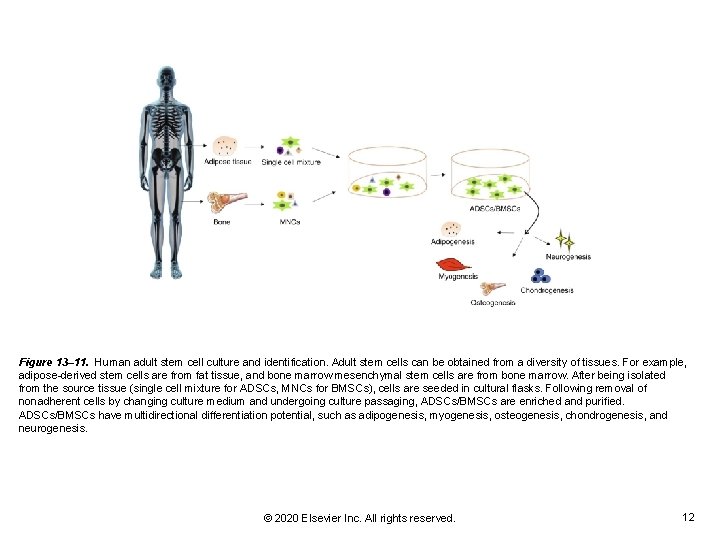
Figure 13– 11. Human adult stem cell culture and identification. Adult stem cells can be obtained from a diversity of tissues. For example, adipose-derived stem cells are from fat tissue, and bone marrow mesenchymal stem cells are from bone marrow. After being isolated from the source tissue (single cell mixture for ADSCs, MNCs for BMSCs), cells are seeded in cultural flasks. Following removal of nonadherent cells by changing culture medium and undergoing culture passaging, ADSCs/BMSCs are enriched and purified. ADSCs/BMSCs have multidirectional differentiation potential, such as adipogenesis, myogenesis, osteogenesis, chondrogenesis, and neurogenesis. © 2020 Elsevier Inc. All rights reserved. 12

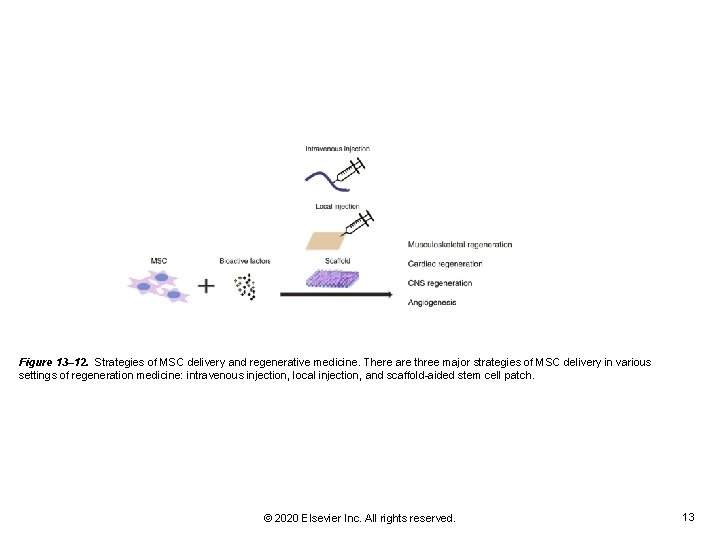
Figure 13– 12. Strategies of MSC delivery and regenerative medicine. There are three major strategies of MSC delivery in various settings of regeneration medicine: intravenous injection, local injection, and scaffold-aided stem cell patch. © 2020 Elsevier Inc. All rights reserved. 13

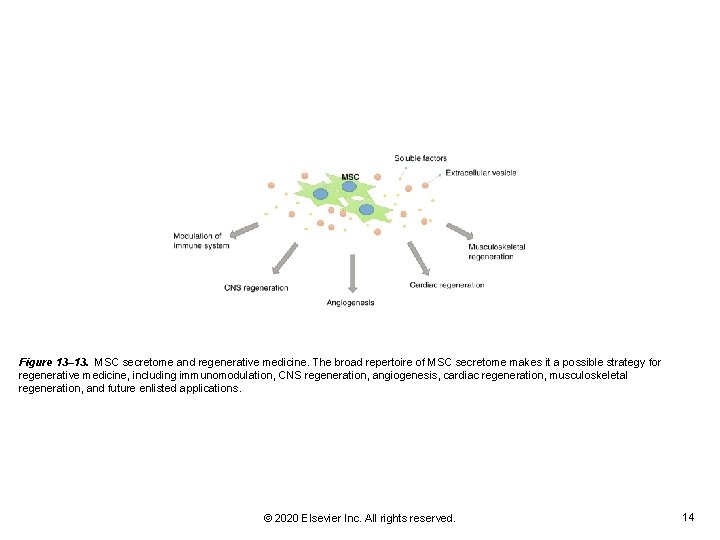
Figure 13– 13. MSC secretome and regenerative medicine. The broad repertoire of MSC secretome makes it a possible strategy for regenerative medicine, including immunomodulation, CNS regeneration, angiogenesis, cardiac regeneration, musculoskeletal regeneration, and future enlisted applications. © 2020 Elsevier Inc. All rights reserved. 14