Focal Lesions in the Cirrhotic Liver Michael P


- Slides: 60

Focal Lesions in the Cirrhotic Liver Michael P. Federle, MD Associate Chair for Education Department of Radiology Stanford University

Focal Lesions in the Cirrhotic Liver • Cysts, hemangiomas, focal fat, confluent fibrosis – Can usually be diagnosed accurately • Hemangiomas shrink and become sclerosed in cirrhotic liver – Often not identified in advanced cirrhosis • Focal fat – Key is out-of-phase MR (focal sign dropout) Brancatelli et al. Radiology 2001; 219: 69 -74

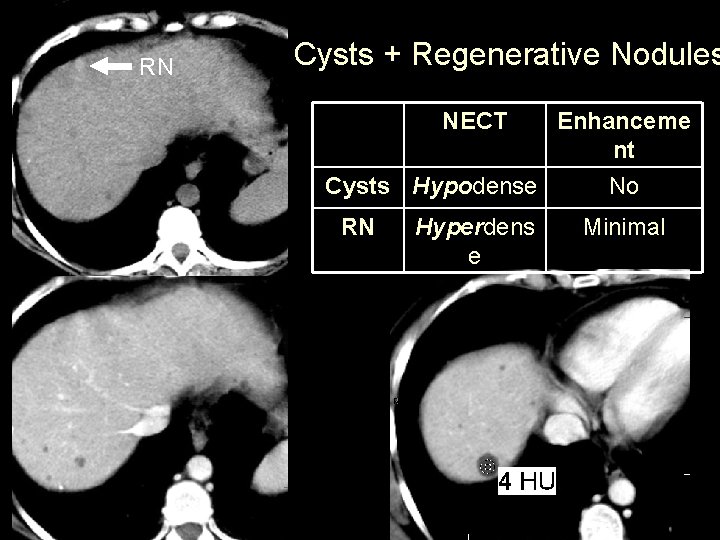
RN Cysts + Regenerative Nodules NECT Enhanceme nt Cysts Hypodense No RN Hyperdens e Minimal

Cavernous Hemangioma • Large ones have typical appearance – Very intense on T 2 WI – Nodular peripheral enhancement • Smaller (“capillary”) hemangiomas – May enhance homogeneously – Can be confused with HCC – Key is remaining isodense with vessels

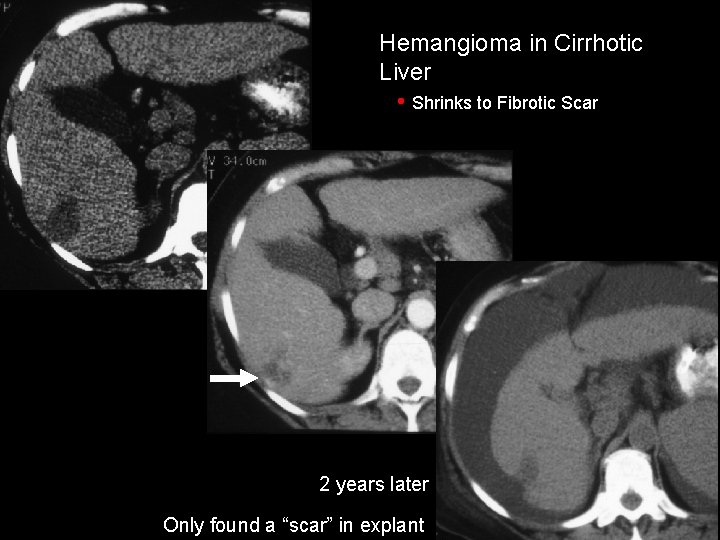
Hemangioma in Cirrhotic Liver • Shrinks to Fibrotic Scar 2 years later Only found a “scar” in explant

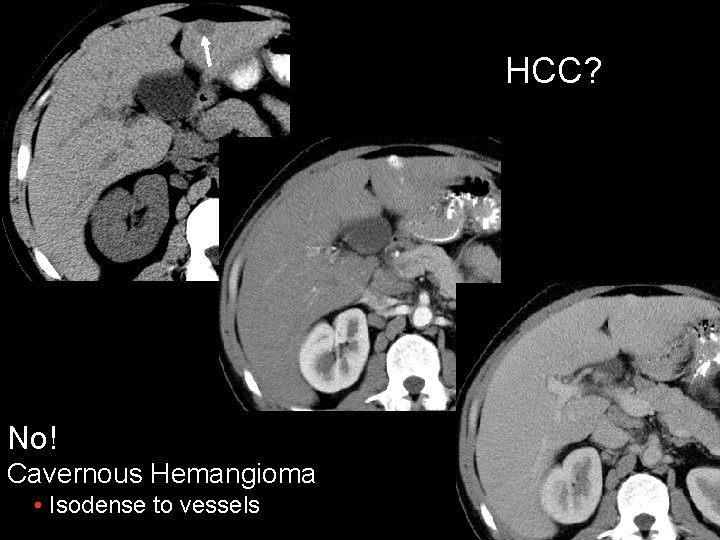
HCC? No! Cavernous Hemangioma • Isodense to vessels

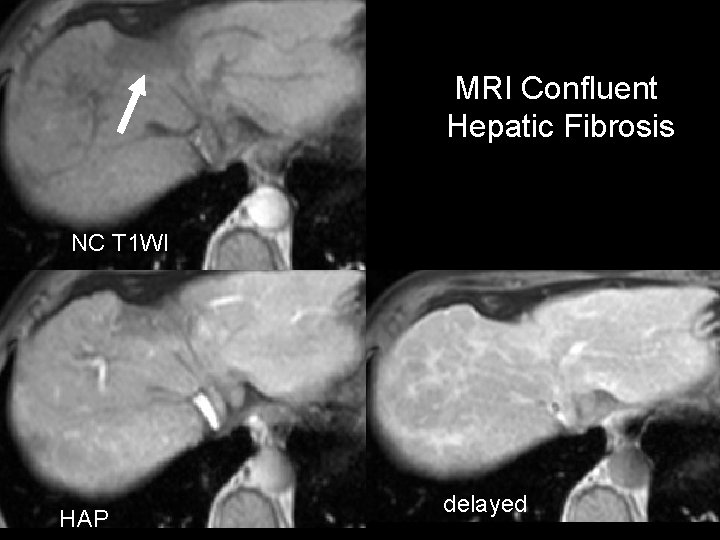
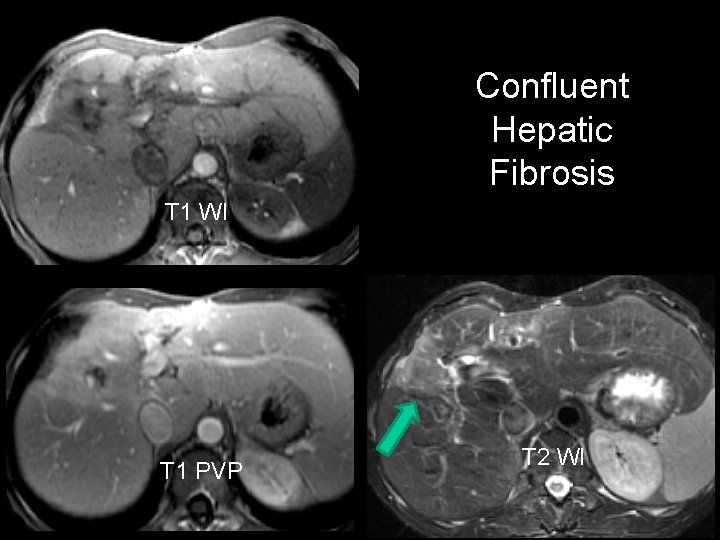
Focal Confluent Fibrosis • Present in ~ 30% of advanced cirrhosis – > 50% of PSC • Most common in anterior + medial segments – Usually wedge-shaped lesion • 80% have focal volume loss – Capsular retraction, crowded vessels • Low density on NCCT – Delayed persistent enhancement • High intensity on T 2 – MR – Can simulate tumor Ohtomo et al. Radiology 1993; 188: 31 -35 Krinsky et al. Radiology 2001; 219: 445 -454

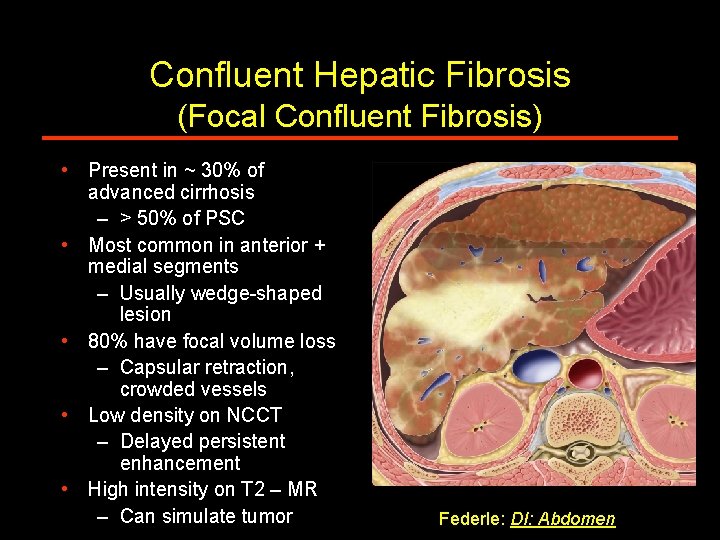
Confluent Hepatic Fibrosis (Focal Confluent Fibrosis) • Present in ~ 30% of advanced cirrhosis – > 50% of PSC • Most common in anterior + medial segments – Usually wedge-shaped lesion • 80% have focal volume loss – Capsular retraction, crowded vessels • Low density on NCCT – Delayed persistent enhancement • High intensity on T 2 – MR – Can simulate tumor Federle: DI: Abdomen

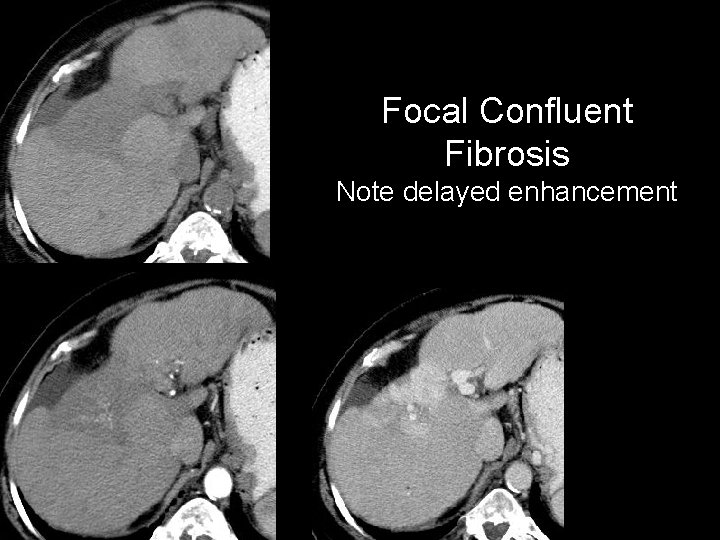
Focal Confluent Fibrosis Note delayed enhancement

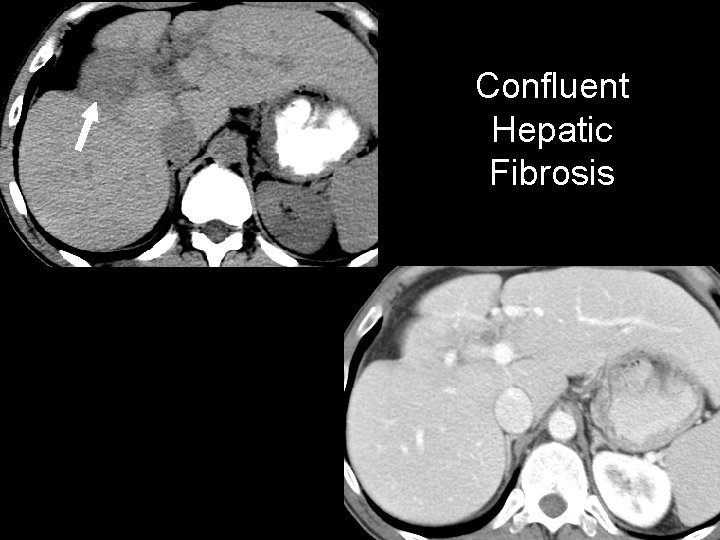
Confluent Hepatic Fibrosis

MRI Confluent Hepatic Fibrosis NC T 1 WI HAP delayed

Confluent Hepatic Fibrosis T 1 WI T 1 PVP T 2 WI

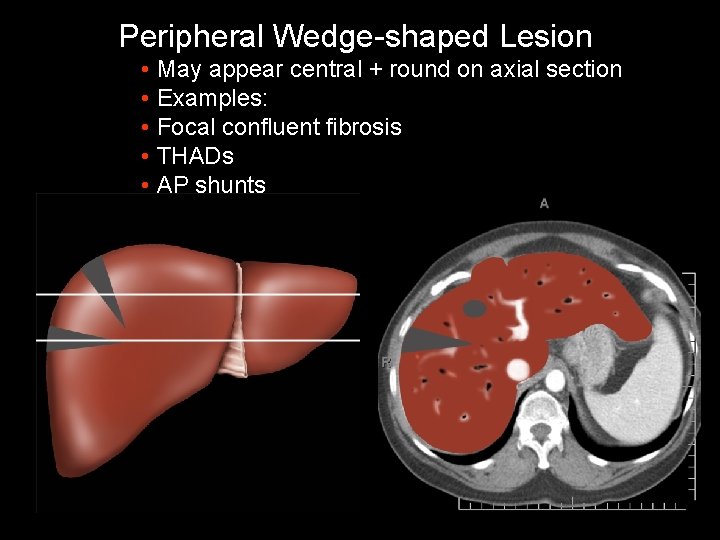
Peripheral Wedge-shaped Lesion • May appear central + round on axial section • Examples: • Focal confluent fibrosis • THADs • AP shunts

Focal Lesions in the Cirrhotic Liver • Regenerative nodules (RN) • Dysplastic nodules • Hepatocellular carcinoma (HCC)

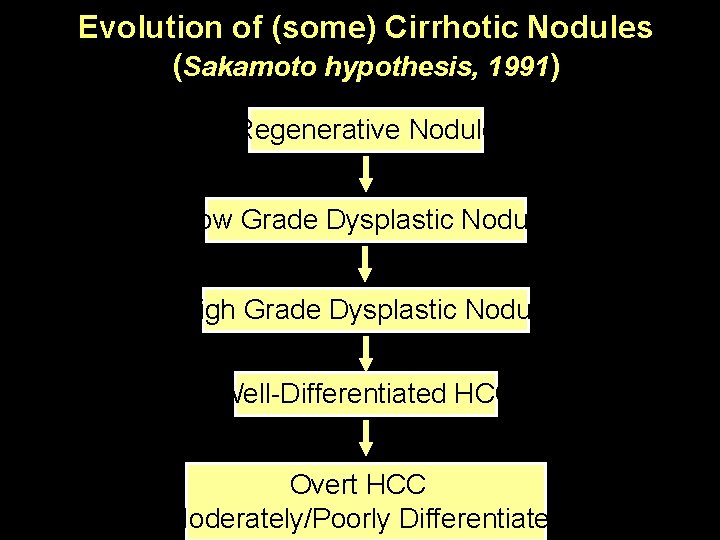
Evolution of (some) Cirrhotic Nodules (Sakamoto hypothesis, 1991) Regenerative Nodule Low Grade Dysplastic Nodule High Grade Dysplastic Nodule Well-Differentiated HCC Overt HCC (Moderately/Poorly Differentiated)

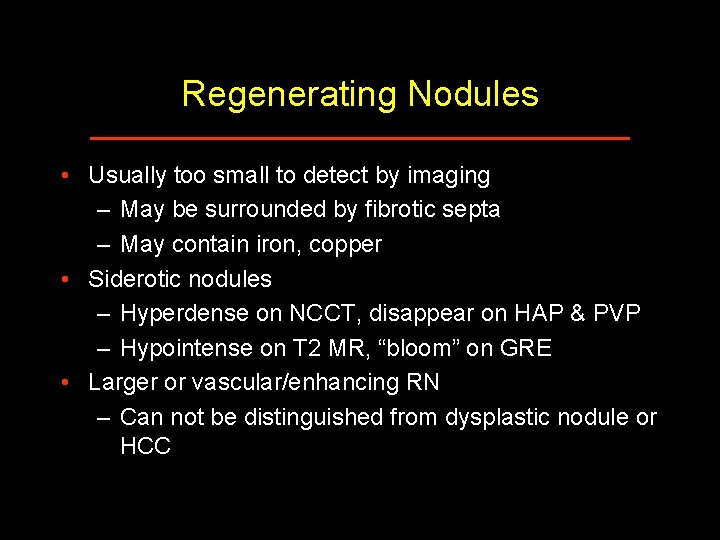
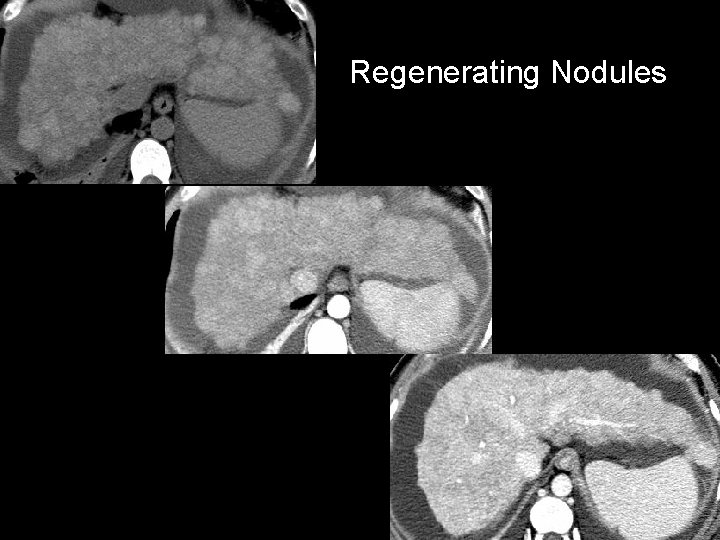
Regenerating Nodules • Usually too small to detect by imaging – May be surrounded by fibrotic septa – May contain iron, copper • Siderotic nodules – Hyperdense on NCCT, disappear on HAP & PVP – Hypointense on T 2 MR, “bloom” on GRE • Larger or vascular/enhancing RN – Can not be distinguished from dysplastic nodule or HCC

Regenerating Nodules

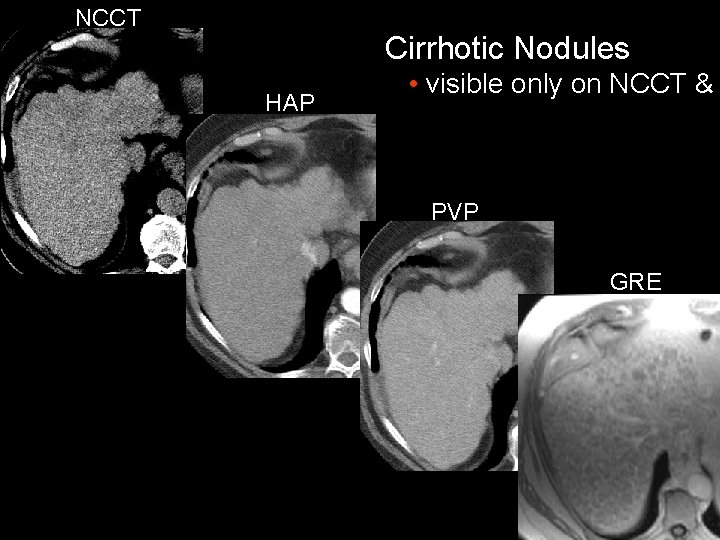
NCCT Cirrhotic Nodules HAP • visible only on NCCT & PVP GRE

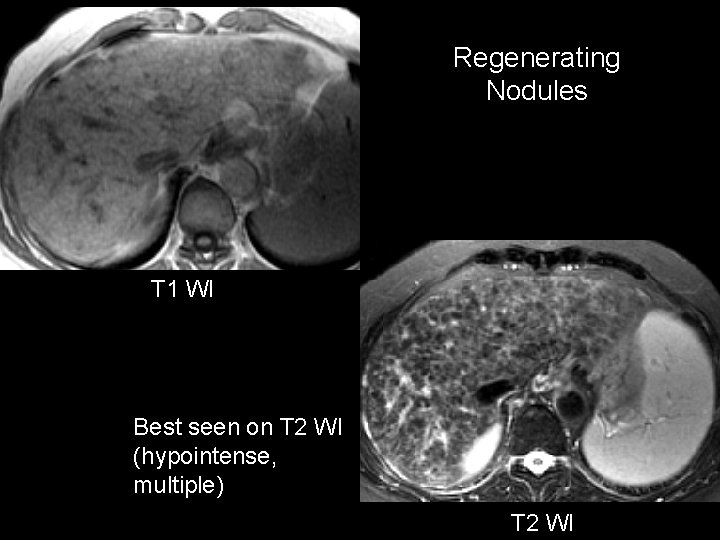
Regenerating Nodules T 1 WI Best seen on T 2 WI (hypointense, multiple) T 2 WI

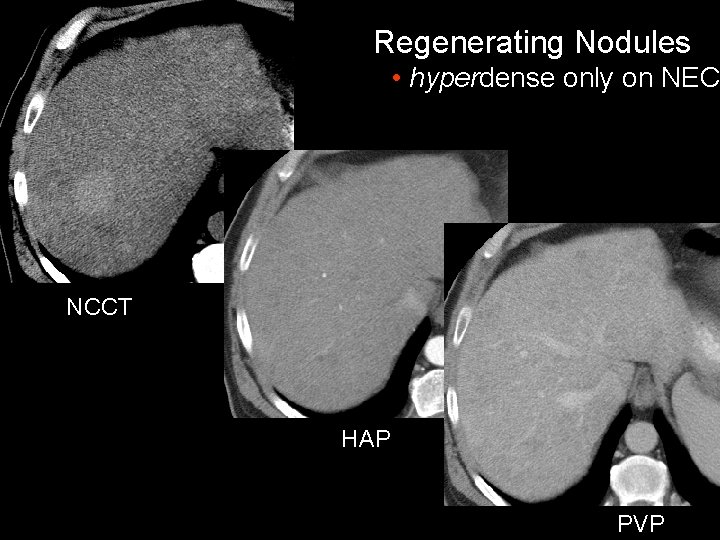
Regenerating Nodules • hyperdense only on NEC NCCT HAP PVP

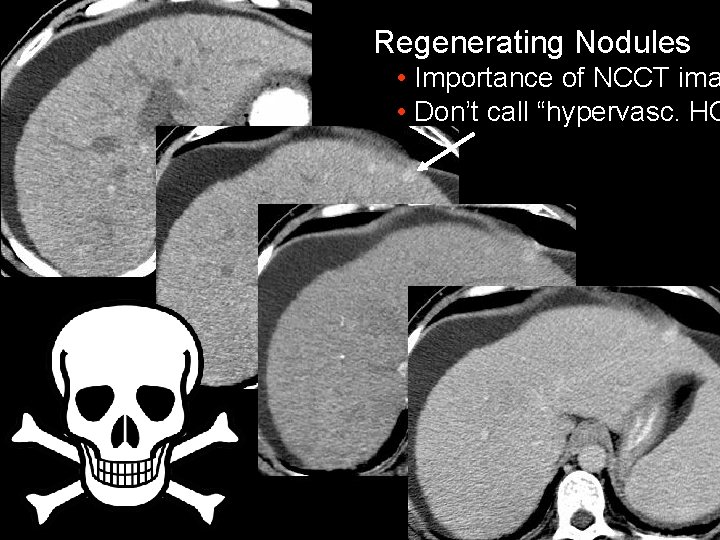
Regenerating Nodules • Importance of NCCT ima • Don’t call “hypervasc. HC

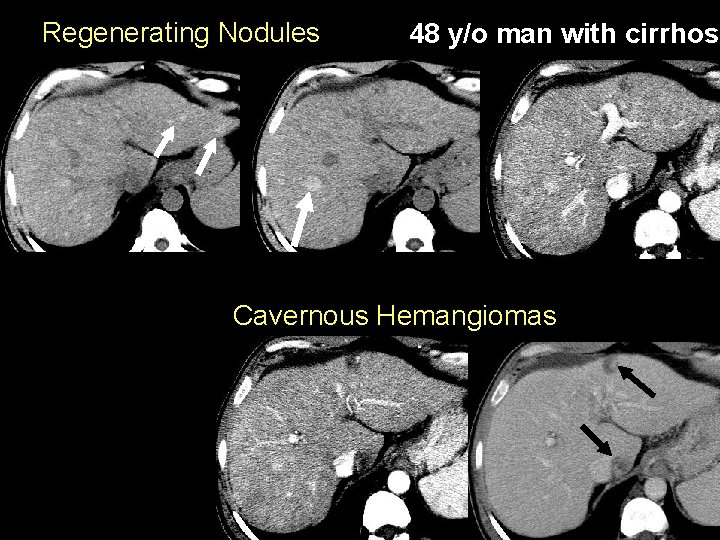
Regenerating Nodules 48 y/o man with cirrhosi Cavernous Hemangiomas

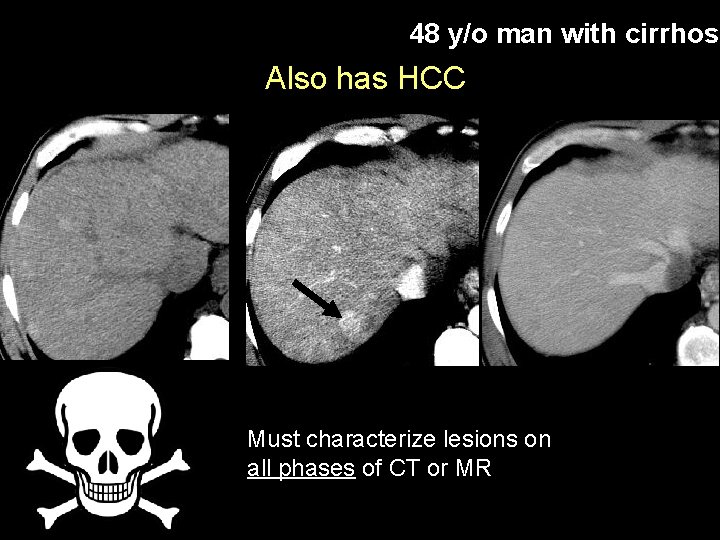
48 y/o man with cirrhosi Also has HCC Must characterize lesions on all phases of CT or MR

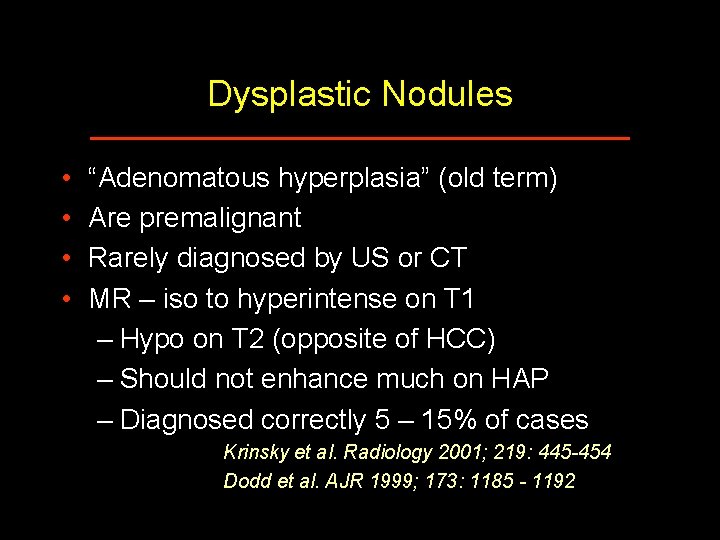
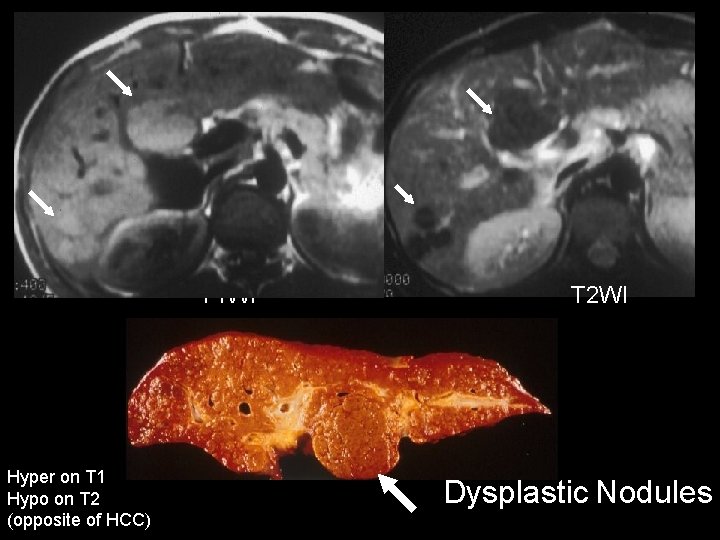
Dysplastic Nodules • • “Adenomatous hyperplasia” (old term) Are premalignant Rarely diagnosed by US or CT MR – iso to hyperintense on T 1 – Hypo on T 2 (opposite of HCC) – Should not enhance much on HAP – Diagnosed correctly 5 – 15% of cases Krinsky et al. Radiology 2001; 219: 445 -454 Dodd et al. AJR 1999; 173: 1185 - 1192

T 1 WI Hyper on T 1 Hypo on T 2 (opposite of HCC) T 2 WI Dysplastic Nodules

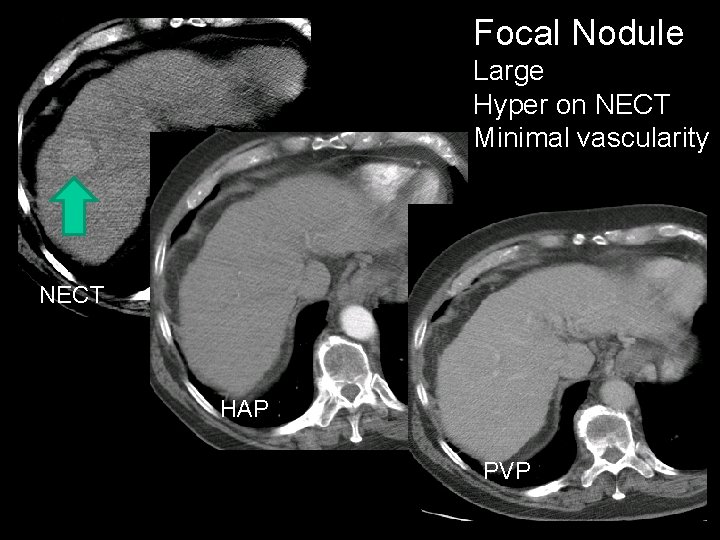
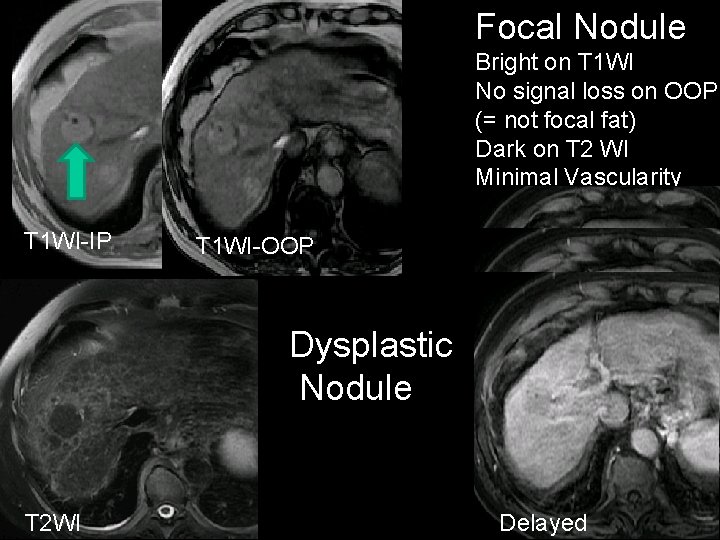
Focal Nodule Large Hyper on NECT Minimal vascularity NECT HAP PVP

Focal Nodule Bright on T 1 WI No signal loss on OOP (= not focal fat) Dark on T 2 WI Minimal Vascularity T 1 WI-IP T 1 WI-OOP Dysplastic Nodule HAP PVP T 2 WI Delayed

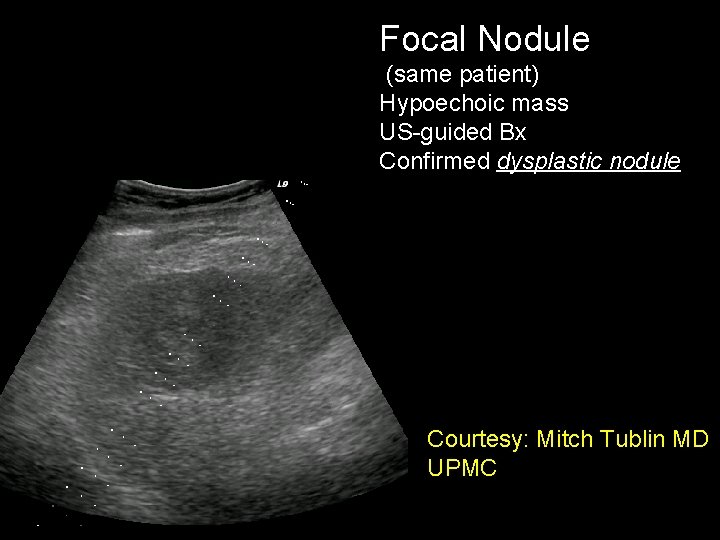
Focal Nodule (same patient) Hypoechoic mass US-guided Bx Confirmed dysplastic nodule Courtesy: Mitch Tublin MD UPMC

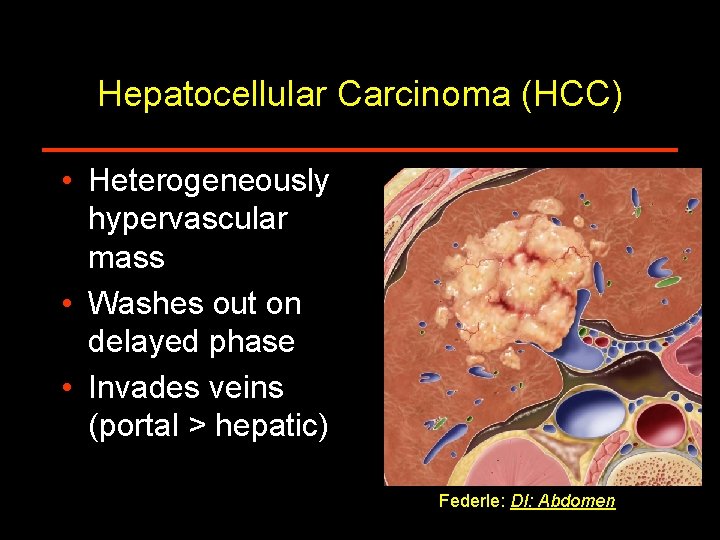
Hepatocellular Carcinoma (HCC) • Heterogeneously hypervascular mass • Washes out on delayed phase • Invades veins (portal > hepatic) Federle: DI: Abdomen

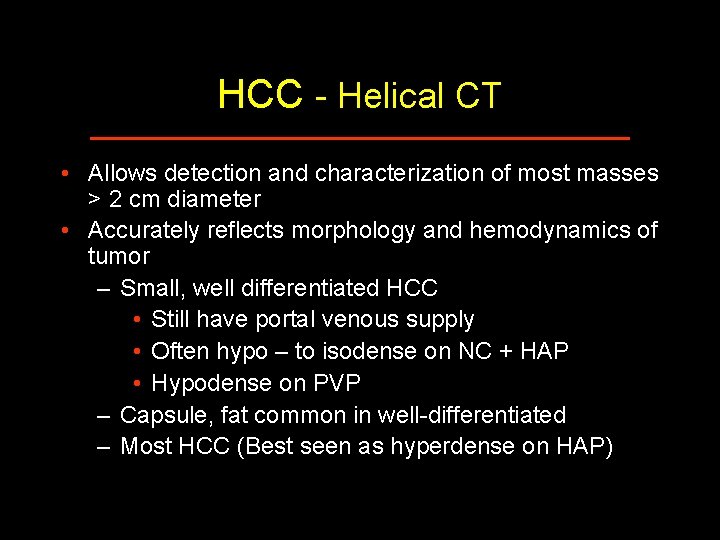
HCC - Helical CT • Main imaging tool in most institutions • Must be multiphasic – Arterial phase ~ 25 – 35 seconds • Dual arterial, or test bolus is ideal – Portal venous ~ 60 – 70 seconds – Noncontrast • Very helpful for RNs, cysts – Delayed or equilibrium • Useful (but hard to justify 4 phase imaging) • Rapid injection (4 or 5 ml/sec); large volume – (2 ml/kg; > 150 ml)

HCC - Helical CT • Allows detection and characterization of most masses > 2 cm diameter • Accurately reflects morphology and hemodynamics of tumor – Small, well differentiated HCC • Still have portal venous supply • Often hypo – to isodense on NC + HAP • Hypodense on PVP – Capsule, fat common in well-differentiated – Most HCC (Best seen as hyperdense on HAP)

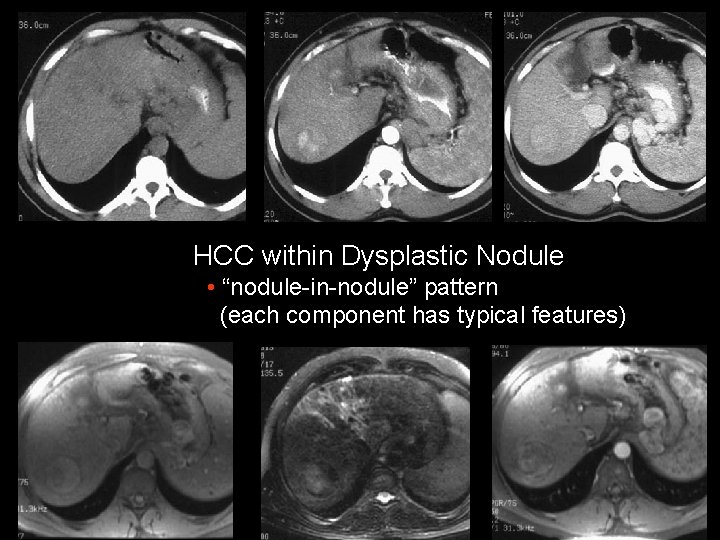
HCC within Dysplastic Nodule • “nodule-in-nodule” pattern (each component has typical features)

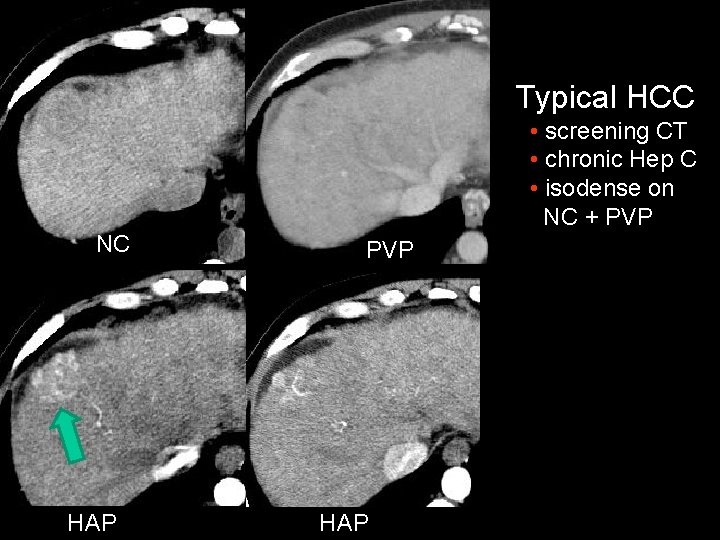
Typical HCC • screening CT • chronic Hep C • isodense on NC + PVP NC HAP PVP HAP

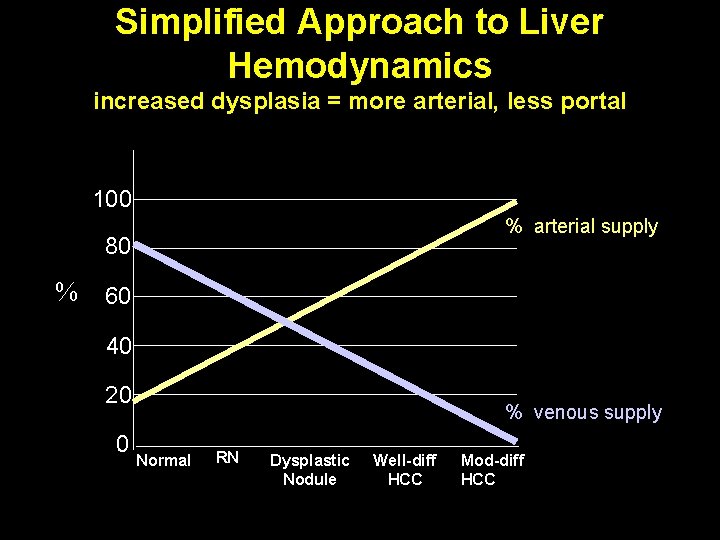
Simplified Approach to Liver Hemodynamics increased dysplasia = more arterial, less portal 100 % arterial supply 80 % 60 40 20 0 % venous supply Normal RN Dysplastic Nodule Well-diff HCC Mod-diff HCC

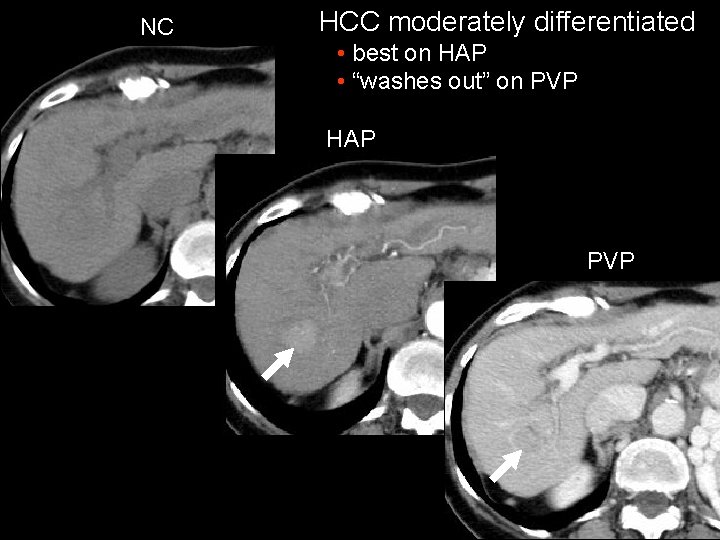
NC HCC moderately differentiated • best on HAP • “washes out” on PVP HAP PVP

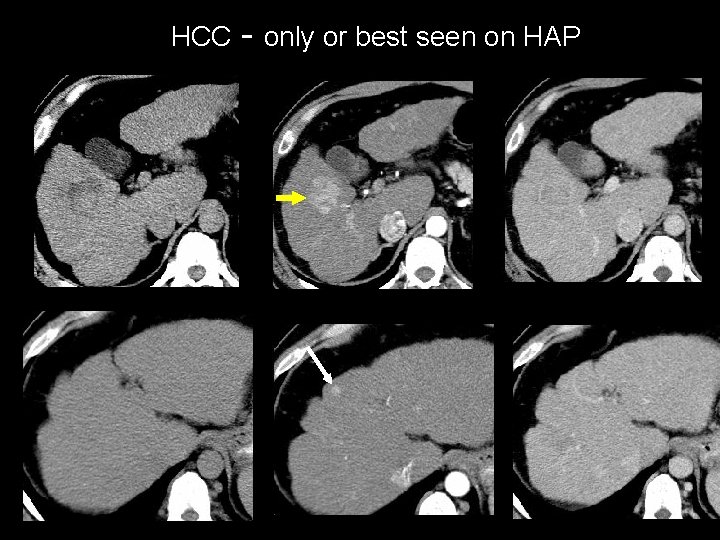
HCC - only or best seen on HAP

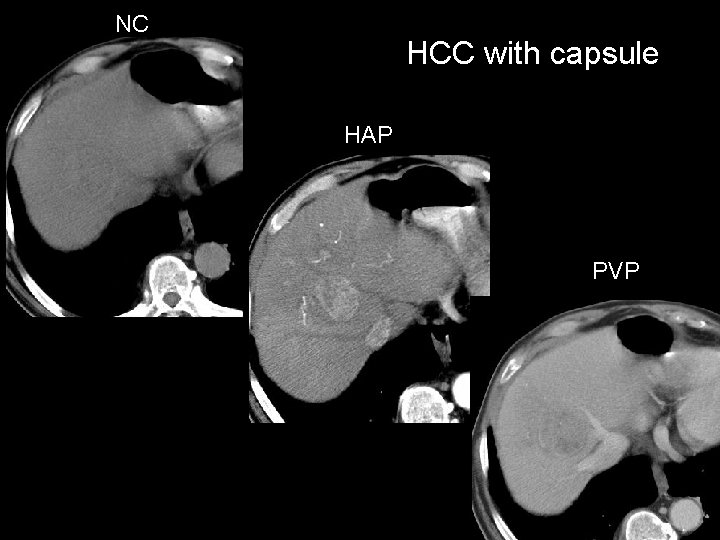
NC HCC with capsule HAP PVP

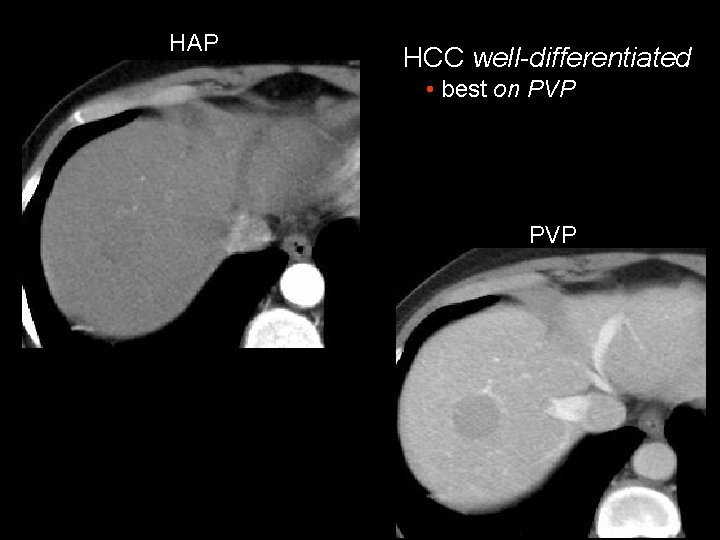
HAP HCC well-differentiated • best on PVP

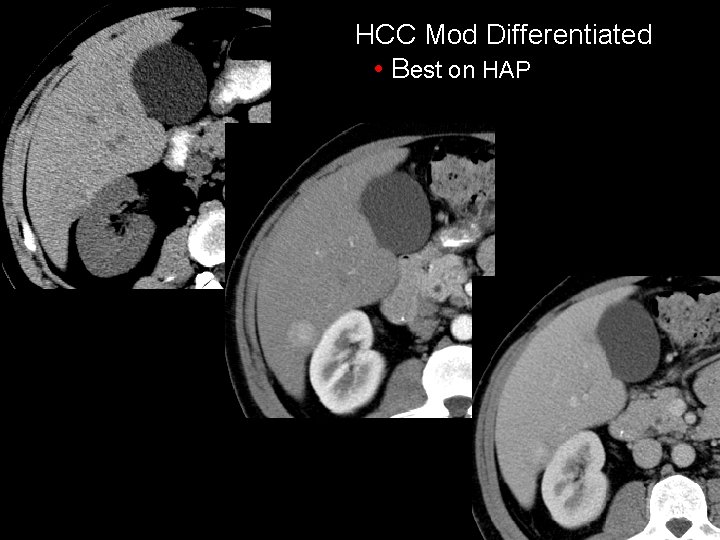
HCC Mod Differentiated • Best on HAP

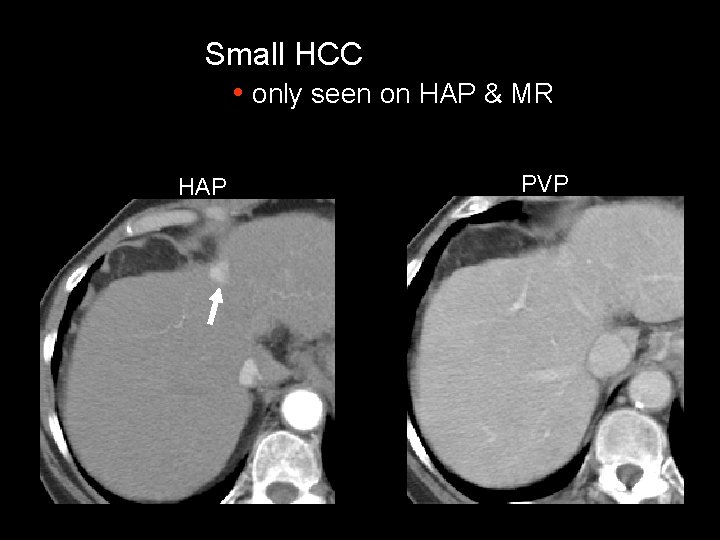
Small HCC • only seen on HAP & MR HAP PVP

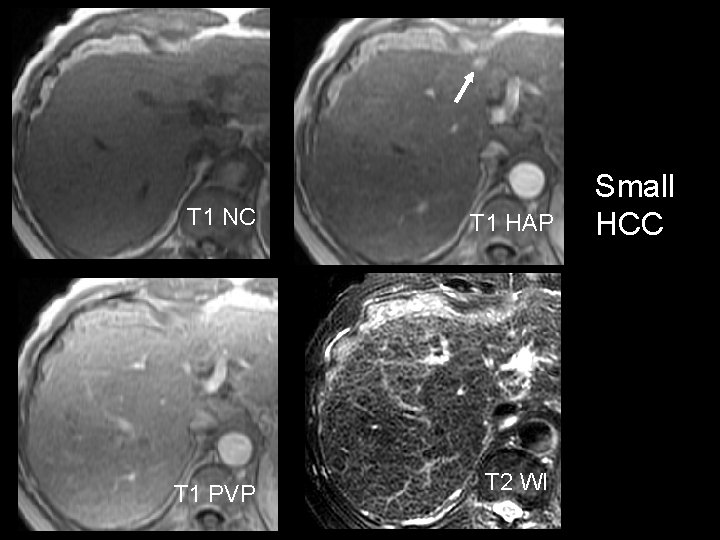
T 1 NC T 1 PVP T 1 HAP T 2 WI Small HCC

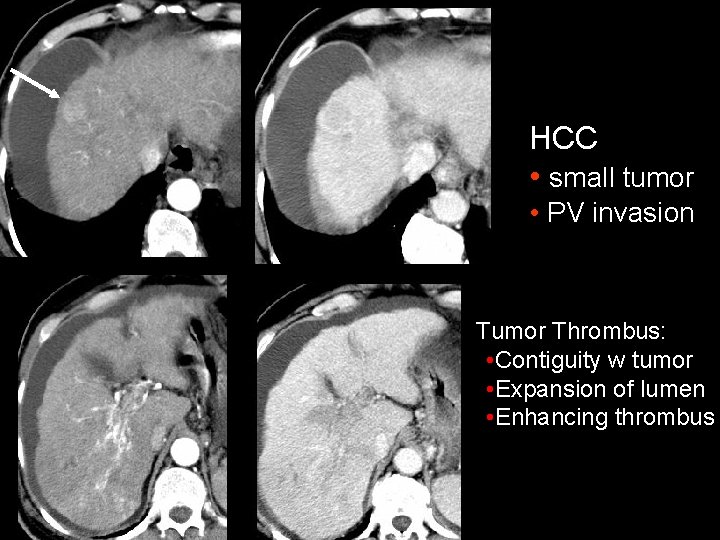
HCC • small tumor • PV invasion Tumor Thrombus: • Contiguity w tumor • Expansion of lumen • Enhancing thrombus

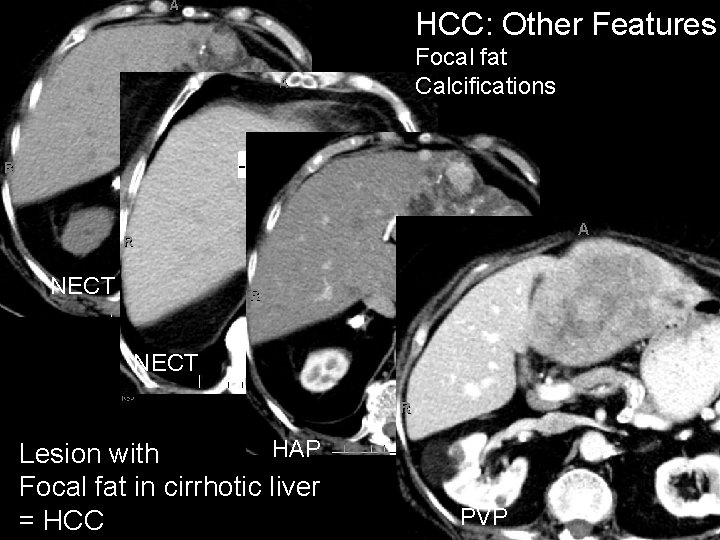
HCC: Other Features Focal fat Calcifications NECT HAP Lesion with Focal fat in cirrhotic liver = HCC PVP

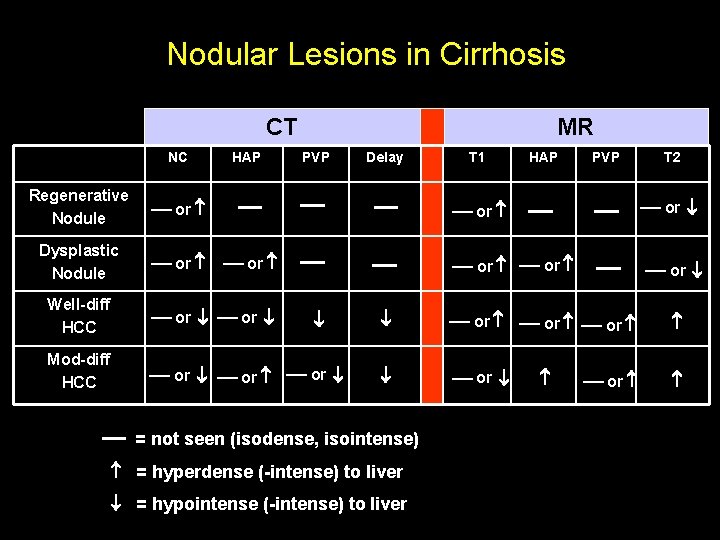
Nodular Lesions in Cirrhosis CT NC Regenerative Nodule or Dysplastic Nodule or HAP MR PVP Delay or Well-diff HCC or or Mod-diff HCC or or or T 1 HAP PVP T 2 or or or = not seen (isodense, isointense) = hyperdense (-intense) to liver = hypointense (-intense) to liver or

HCC - Helical CT Accuracy • Good for large tumors • Challenging in screening population (asymptomatic, normal tumor markers) • We miss (false + and neg) small HCCs (<2 cm) frequently • However, we usually (> 95%, UPMC data) accurately guide Rx – Decision for follow-up, ablation, TACE, transplantation

HCC - Helical CT Accuracy • Multidetector CT and dual arterial phase imaging • Sensitivity (86%), positive pred value (92%) – Mean size of HCC (22 mm) • Much better results than other reports Murakami et al. Radiology 2001; 218: 763 -767

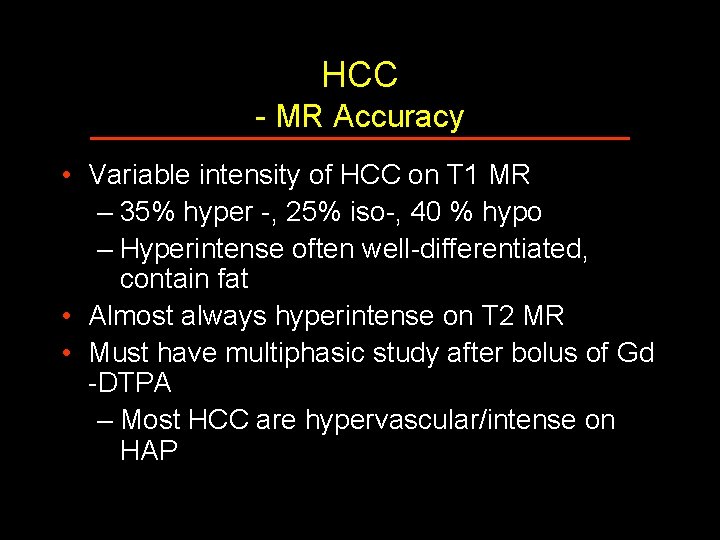
HCC - MR Accuracy • Variable intensity of HCC on T 1 MR – 35% hyper -, 25% iso-, 40 % hypo – Hyperintense often well-differentiated, contain fat • Almost always hyperintense on T 2 MR • Must have multiphasic study after bolus of Gd -DTPA – Most HCC are hypervascular/intense on HAP

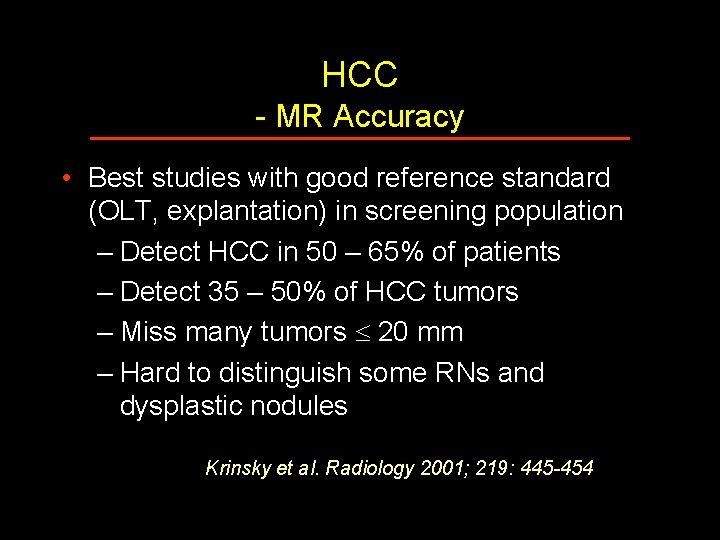
HCC - MR Accuracy • Best studies with good reference standard (OLT, explantation) in screening population – Detect HCC in 50 – 65% of patients – Detect 35 – 50% of HCC tumors – Miss many tumors 20 mm – Hard to distinguish some RNs and dysplastic nodules Krinsky et al. Radiology 2001; 219: 445 -454

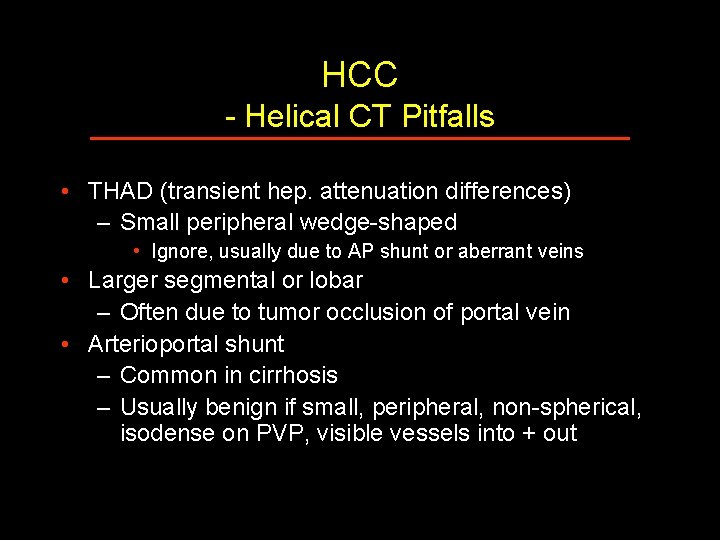
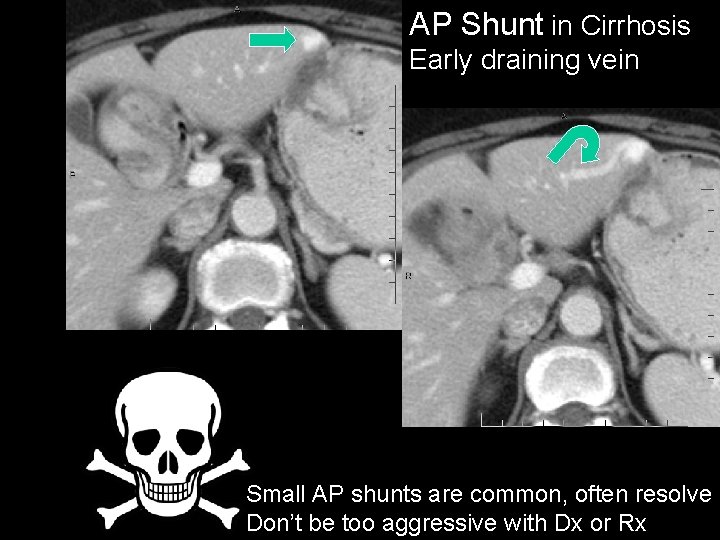
HCC - Helical CT Pitfalls • THAD (transient hep. attenuation differences) – Small peripheral wedge-shaped • Ignore, usually due to AP shunt or aberrant veins • Larger segmental or lobar – Often due to tumor occlusion of portal vein • Arterioportal shunt – Common in cirrhosis – Usually benign if small, peripheral, non-spherical, isodense on PVP, visible vessels into + out

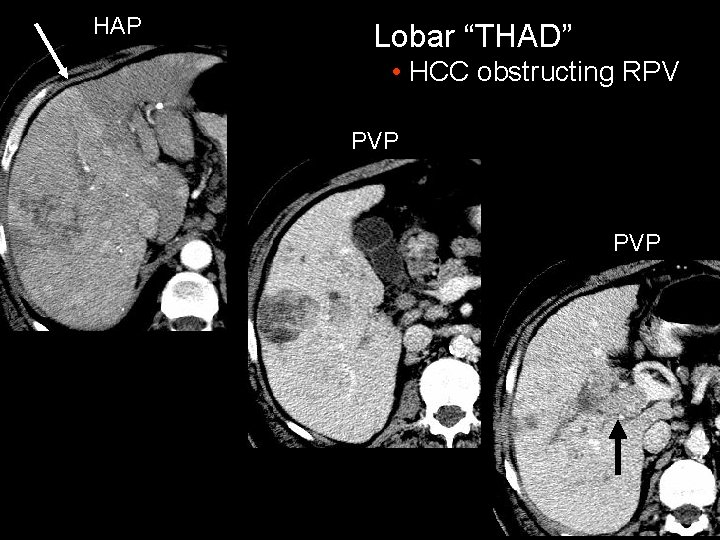
HAP Lobar “THAD” • HCC obstructing RPV PVP

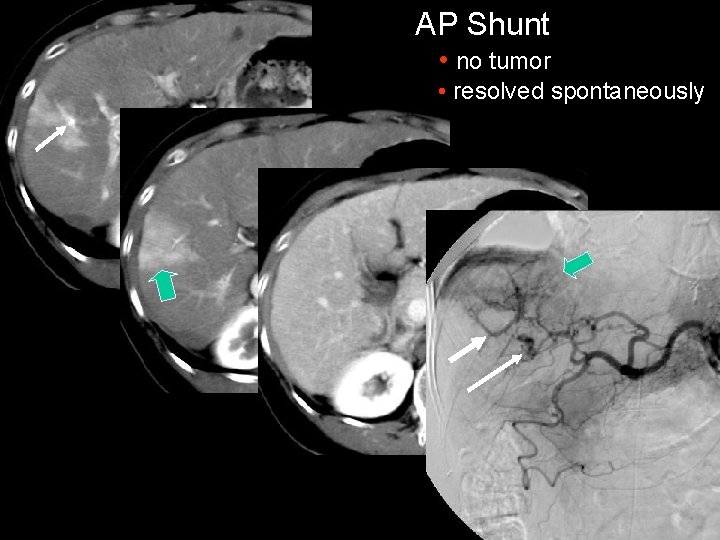
AP Shunt • no tumor • resolved spontaneously

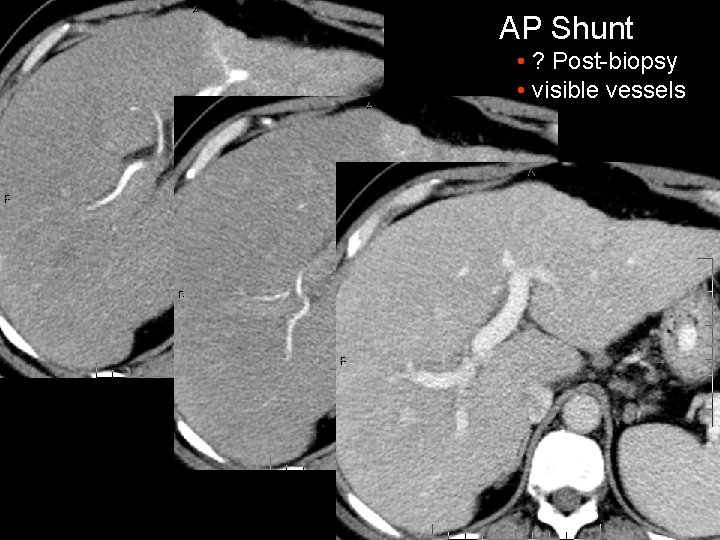
AP Shunt • ? Post-biopsy • visible vessels

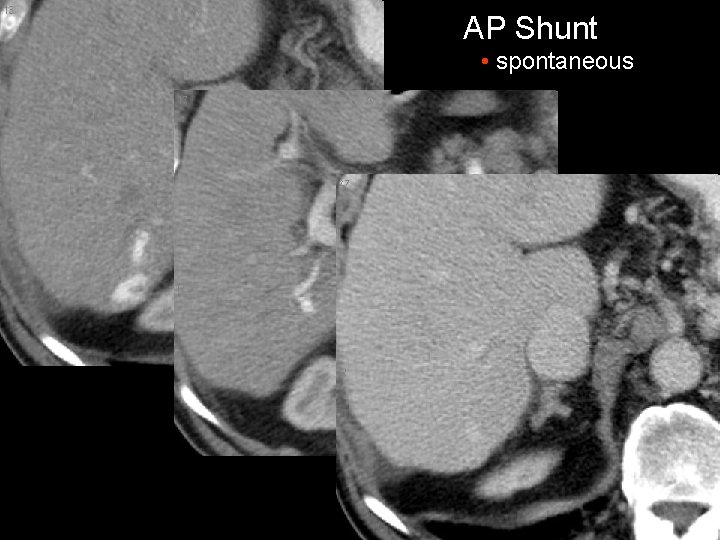
AP Shunt • spontaneous

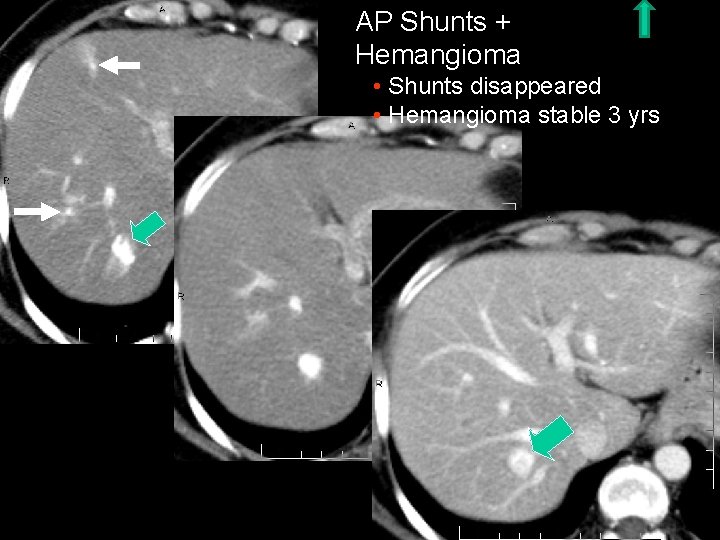
AP Shunts + Hemangioma • Shunts disappeared • Hemangioma stable 3 yrs

AP Shunt in Cirrhosis Early draining vein Small AP shunts are common, often resolve Don’t be too aggressive with Dx or Rx

HCC - Helical CT vs MR • Comparable performance • MR preference – Contrast allergy – Known steatosis • CT preference – Ascites, unstable, tachypneic patient • Both are evolving and improving (but often performed/interpreted poorly)

Tumor Markers for HCC • Pitt Experience with 430 transplant recipients – Excluding 2 patients with HCC + markedly AFP – No significant difference in serum AFP in HCC, non-HCC groups – AFP often normal in small HCC – AFP often elevated in flare of hepatitis Peterson et al. Radiology 2000; 217: 743 -749

Screening Recommendation for Known Cirrhosis • • AFP and PIVKA II – every 3 months Ultrasonography – every 3 or 4 months CT or MR – every 12 months (for chronic hepatitis without cirrhosis, extend intervals) • (for high clinical suspicion or indeterminate lesion, shorten interval)

Summary • US, CT, MR all useful in evaluation of cirrhosis • Large and symptomatic HCCs are easily detected and staged • Small HCCs in a screening population are more challenging – Some overlap in appearance of regenerative + dysplastic nodules + HCC

Summary • Optimal CT + MR techniques are key – Must include multiple phases, rapid bolus contrast administration • Image-guided Bx and angiography often necessary