Cairo Univ Faculty of Vet Med Pathology Dept












- Slides: 28

Cairo Univ. Faculty of Vet. Med. Pathology Dept. Prepared by: Sarah Youssry El-Sayed (06618) Supervised by: Prof. Dr. Kawkab

I would like to express my deep gratitude to the stuff of Pathology Department especially the head of the department and Prof. Kwkab for their cooperation.

Introduction Foot-and-mouth disease (FMD) is a highly contagious, severe, clinically acute, vesicular disease of the members of the order Arteriodactyla, that is, cloven-hoofed animals, including domesticated ruminants and pigs, and more than 70 wildlife species. FMD is a problem of worldwide concern, as it is enzootic in large areas of Africa, Asia, Europe, and South America (except the Guyanas and Chile). Foot-and-mouth disease virus (FMDV) is classified within the Aphthovirus genus of the Picornaviridae family. The 7 principal FMD is characterized by the formation of vesicles in the mouth and on the feet, teats, and mammary glands. In general, sheep and goats are less susceptible than other ruminants, and the clinical signs are often mild. Lesions are often seen on dental pads and the dorsum of the tongue, but they are also seen on the lips, gums, and cheeks and sometimes on the hard palate. 2, 4 The disease is notable for high mortality except in suckling animals; however, morbidity is very high. 4 The mortality in young animals, in particular lambs and piglets, may be due to acute myocarditis. Myocarditis is considered a fatal form of FMD that occurs without vesiculation in young animals. 2, 4 The residual lesions in older cattle qualify as myocarditis with a significant inflammatory response. 39 However, the significance of acute myocarditis in the spread of FMDV has not been studied in detail. 2 The virus can usually be isolated from myocardia in areas containing necrotic myocytes that are infiltrated with mononuclear cells. 13

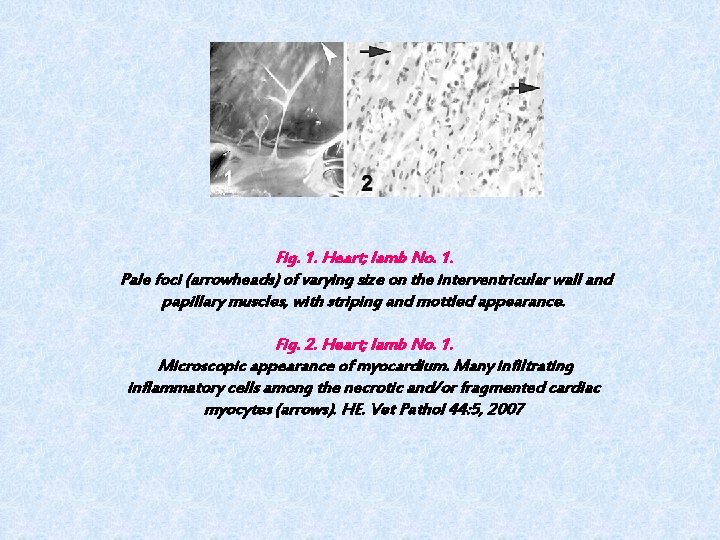
Fig. 1. Heart; lamb No. 1. Pale foci (arrowheads) of varying size on the interventricular wall and papillary muscles, with striping and mottled appearance. Fig. 2. Heart; lamb No. 1. Microscopic appearance of myocardium. Many infiltrating inflammatory cells among the necrotic and/or fragmented cardiac myocytes (arrows). HE. Vet Pathol 44: 5, 2007

Historyof disease The disease was initially described in the 16 th century and was the first animal pathogen identified as a virus. Recent FMD outbreaks in developed countries and their significant economic impact have increased the concern of governments worldwide. This review describes the reemergence of FMD in developed countries that had been disease free for many years and the effect that this had on disease control strategies. Definition Foot-and-mouth disease (FMD) is a highly contagious disease of clovenhoofed animals. Host a disease of domestic livestock - cattle, sheep, pigs and goats but it is highly contagious disease of cloven-hoofed animals. The Disease in Susceptible Wild Mammals FMD may cause serious disease in wild animals. 50% of a population of Gazella gazella - Mountain gazelle died when FMD went through the population in Israel. Ten percent of Odocoileus hemionus - Mule deer in California were found to have FMD lesions in the last outbreak in the USA. In Britain, hedgehogs (Erinaceus europaeus - West European hedgehog) have been found with serious and fatal disease while deer on and near Infected Premises have been seen lame and with typical FMD lesions. However, various species also may develop only mild clinical signs, or seroconvert without obvious illness developing. Transfer of the virus between wild and domestic animals is most likely where there is close contact between the animals. Pets such as cats, dogs and rabbits are not normally at risk of infection with FMD unless the virus is actually injected into them. Horses do not get the disease. All these species, as with all other species including humans, can spread from one place to another for

Cause Foot-and-mouth disease virus (FMDV) is a member of the genus Aphthovirus in the family Picornaviridae, all single-stranded, nonenveloped, positive sense RNA viruses. Foot-and-mouth Mode of transmission: Through urine&feacesof infected animal&in dust&by inhalation&by contact. Wild birds, rodents, and invertebrates such as flies and ticks may all carry virus from one place to another. Rats may play a larger role in spread of the disease as they can become infected naturally and shed virus. Pathogenesis Infection begins when inhaled viruses reach the lungs of susceptible animals. After a period of replication in the bronchioles, viremia ensues. Viremia is accompanied by fever, and within 1 to 2 days, the virus is established at multiple epithelial sites. The virus replicates in rafts of cells in the stratum spinosum. The infection is cytolytic, with resulting cavity formation within the stratum spinosum. Fluid-laden vesicles are seen grossly at coronary bands, interdigital clefts, tongue, palate, snout, and, in lactating animals, teats. It is thought that the virus is transported into the stratum spinosum via Langerhans cells and that the infected "raft" of cells comprises all the keratinoyctes contacted by one infected Langerhans cell. 6

Under natural conditions, the most common form of transmission is by aerosol, with high concentrations of infectious particles exhaled by an animal in the acute phase of the disease being carried on the air to the respiratory tract of a susceptible animal. More than any other infected species, pigs produce enormous quantities of virus, with over a hundred million infectious virus particles exhaled per day. Consequently, pigs are often referred to as the "amplifier hosts" of FMD. The virus is also present in vesicular fluid and saliva, and at the peak of infection can be found in blood and tissues of the affected animal. 4 Although the virus is readily inactivated in muscle under post mortem conditions because of the rapid drop in p. H, it may survive in pockets of lymphoid tissue and bone marrow. Most new outbreaks begin with animal contact or consumption of animal byproducts. Illegally imported, virus-contaminated meat products fed as garbage to pigs have caused many new outbreaks in the world, and are suspected as the cause of the epidemic in eastern Russia in 20009 and in the UK in 2001. 10 Although most countries require that garbage fed to pigs be thoroughly cooked, which inactivates the virus, not all garbage-feeding operations adhere to this practice. Introduction of healthy carrier animals has also been responsible for sparking outbreaks. Recovered pigs clear the infection completely, but in ruminant species, the virus may be harbored in the oropharynx of a percentage of recovered animals, which become healthy carriers, with potential spread to new areas. 11 Also, infected sheep and goats, which often show very few clinical signs of the disease, may be moved to new areas, generating an outbreak such as the one that occurred in Greece in 1995. 7 The virus survives in the environment fairly well and can persist for up to 1 month under the favorable conditions of high humidity, cool temperature, and appropriate p. H (>6. 0 and <9. 0). Transport of contaminated hay into the Republic of Korea and Japan is thought to be the cause of the outbreaks there in 2000. 9 In addition, veterinary equipment and vehicles are notorious for acting as fomites to carry FMDV from one premise to another. People coming to and going from an infected farm can also act as fomites, carrying the virus on their clothes or shoes. It has been shown that FMDV can reside passively in the human nasal passages for 24 hours and so be carried

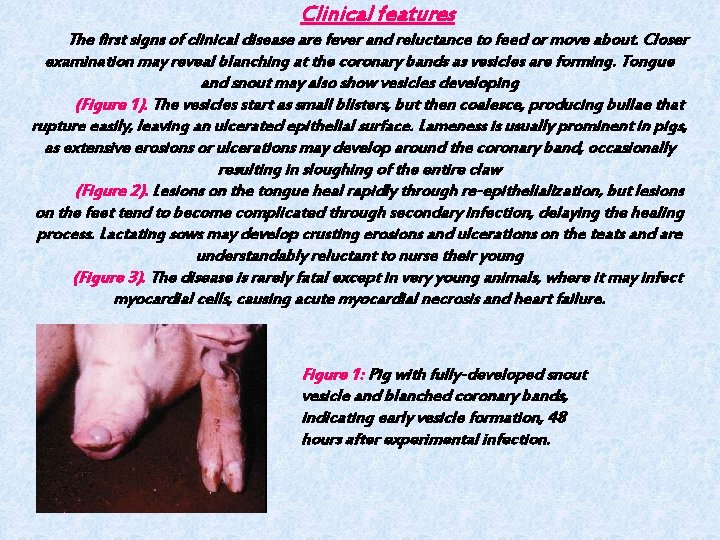
Clinical features The first signs of clinical disease are fever and reluctance to feed or move about. Closer examination may reveal blanching at the coronary bands as vesicles are forming. Tongue and snout may also show vesicles developing (Figure 1). The vesicles start as small blisters, but then coalesce, producing bullae that rupture easily, leaving an ulcerated epithelial surface. Lameness is usually prominent in pigs, as extensive erosions or ulcerations may develop around the coronary band, occasionally resulting in sloughing of the entire claw (Figure 2). Lesions on the tongue heal rapidly through re-epithelialization, but lesions on the feet tend to become complicated through secondary infection, delaying the healing process. Lactating sows may develop crusting erosions and ulcerations on the teats and are understandably reluctant to nurse their young (Figure 3). The disease is rarely fatal except in very young animals, where it may infect myocardial cells, causing acute myocardial necrosis and heart failure. Figure 1: Pig with fully-developed snout vesicle and blanched coronary bands, indicating early vesicle formation, 48 hours after experimental infection.

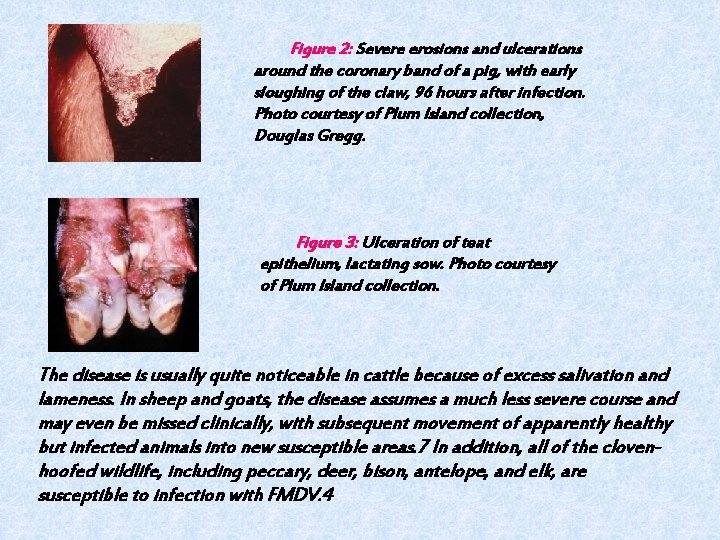
Figure 2: Severe erosions and ulcerations around the coronary band of a pig, with early sloughing of the claw, 96 hours after infection. Photo courtesy of Plum Island collection, Douglas Gregg. Figure 3: Ulceration of teat epithelium, lactating sow. Photo courtesy of Plum Island collection. The disease is usually quite noticeable in cattle because of excess salivation and lameness. In sheep and goats, the disease assumes a much less severe course and may even be missed clinically, with subsequent movement of apparently healthy but infected animals into new susceptible areas. 7 In addition, all of the clovenhoofed wildlife, including peccary, deer, bison, antelope, and elk, are susceptible to infection with FMDV. 4

Diagnosis It is not possible to diagnose FMD solely on the basis of the gross or histologic appearance of the disease. Grossly, all diseases causing oral, snout, pedal, or teat erosions should be considered in the differential diagnosis of FMD. Histologically, all vesicular diseases (FMD, swine vesicular disease, vesicular stomatitis) have similar morphologic characteristics and even in the vesicular stage cannot be differentiated from one another microscopically. All are characterized by intra- and intercellular edema in large groups of cells within the stratum spongiosum. Once the vesicles have ruptured, the histology of vesicular diseases is very similar to many erosive and ulcerative diseases. Laboratory confirmation of an outbreak is essential. In most countries, the appearance of any vesicular disease must be reported to the authorities for adequate investigation. Veterinarians trained by the USDA are responsible for inspecting clinically affected animals, collecting samples, and sending these samples to federal laboratories for diagnosis. Because of the regulatory implications of the presence of FMD, all laboratory testing is done by the federal government at the Foreign Animal Disease Diagnostic Laboratory, Plum Island. A number of laboratory tests are used for detection of FMDV. In acutely infected animals, ideal materials to collect include vesicular fluid within intact vesicles, or epithelial tags surrounding ruptured vesicles. An ELISA antigen assay may be used for rapid detection of FMDV if there is sufficient virus in the sample. If the sample is inadequate or the test results are inconclusive, cell cultures are inoculated. When cytopathic effects are detected in cell culture, the fluids are tested by ELISA. Virus neutralization and ELISA are used to test for serum antibodies: both of these serological tests are serotype specific

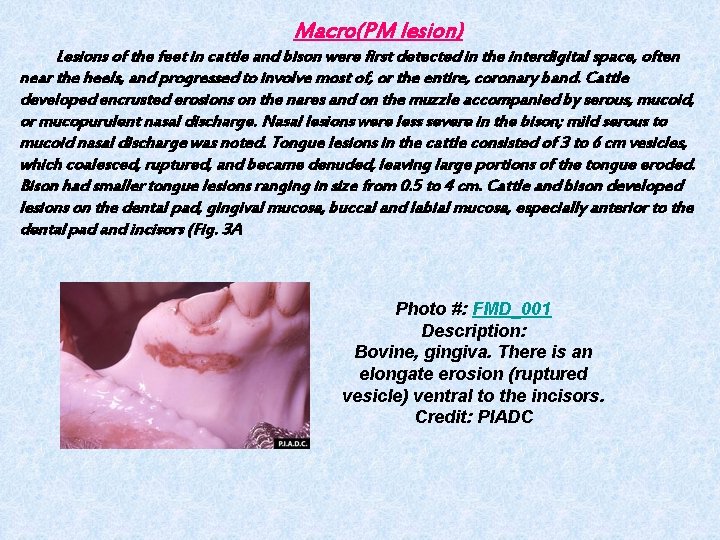
Macro(PM lesion) Lesions of the feet in cattle and bison were first detected in the interdigital space, often near the heels, and progressed to involve most of, or the entire, coronary band. Cattle developed encrusted erosions on the nares and on the muzzle accompanied by serous, mucoid, or mucopurulent nasal discharge. Nasal lesions were less severe in the bison; mild serous to mucoid nasal discharge was noted. Tongue lesions in the cattle consisted of 3 to 6 cm vesicles, which coalesced, ruptured, and became denuded, leaving large portions of the tongue eroded. Bison had smaller tongue lesions ranging in size from 0. 5 to 4 cm. Cattle and bison developed lesions on the dental pad, gingival mucosa, buccal and labial mucosa, especially anterior to the dental pad and incisors (Fig. 3 A Photo #: FMD_001 Description: Bovine, gingiva. There is an elongate erosion (ruptured vesicle) ventral to the incisors. Credit: PIADC

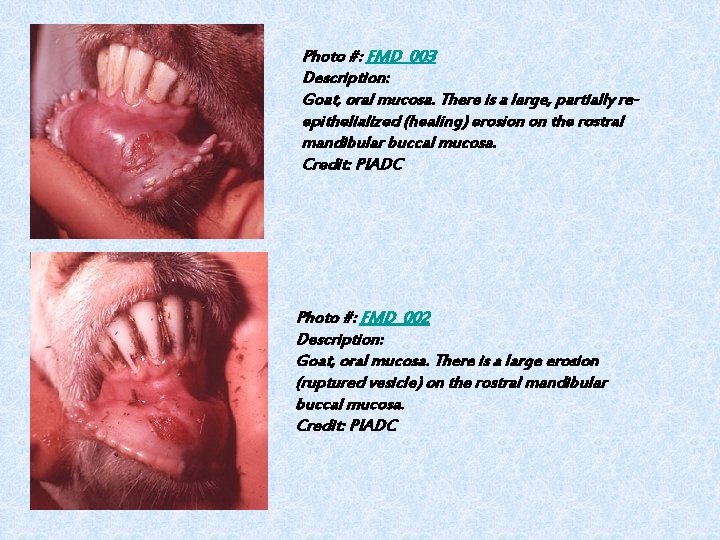
Photo #: FMD_003 Description: Goat, oral mucosa. There is a large, partially reepithelialized (healing) erosion on the rostral mandibular buccal mucosa. Credit: PIADC Photo #: FMD_002 Description: Goat, oral mucosa. There is a large erosion (ruptured vesicle) on the rostral mandibular buccal mucosa. Credit: PIADC

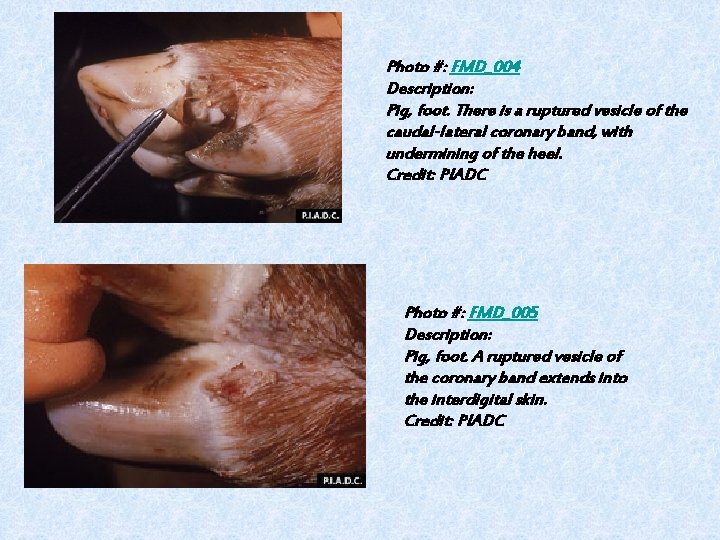
Photo #: FMD_004 Description: Pig, foot. There is a ruptured vesicle of the caudal-lateral coronary band, with undermining of the heel. Credit: PIADC Photo #: FMD_005 Description: Pig, foot. A ruptured vesicle of the coronary band extends into the interdigital skin. Credit: PIADC

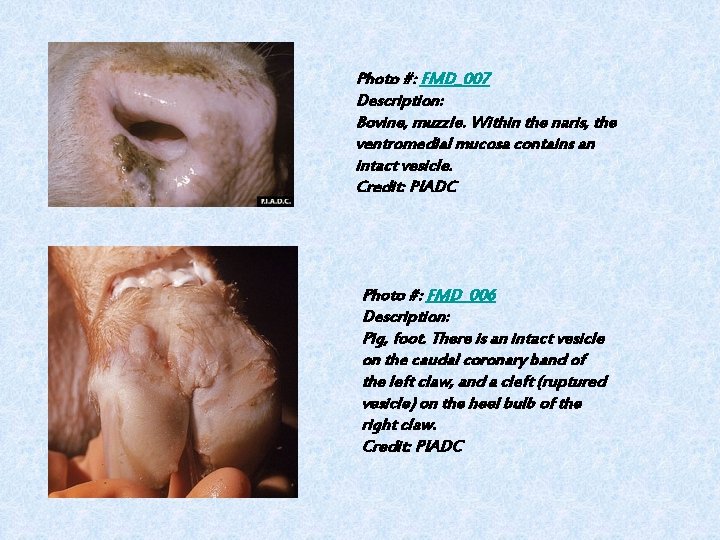
Photo #: FMD_007 Description: Bovine, muzzle. Within the naris, the ventromedial mucosa contains an intact vesicle. Credit: PIADC Photo #: FMD_006 Description: Pig, foot. There is an intact vesicle on the caudal coronary band of the left claw, and a cleft (ruptured vesicle) on the heel bulb of the right claw. Credit: PIADC

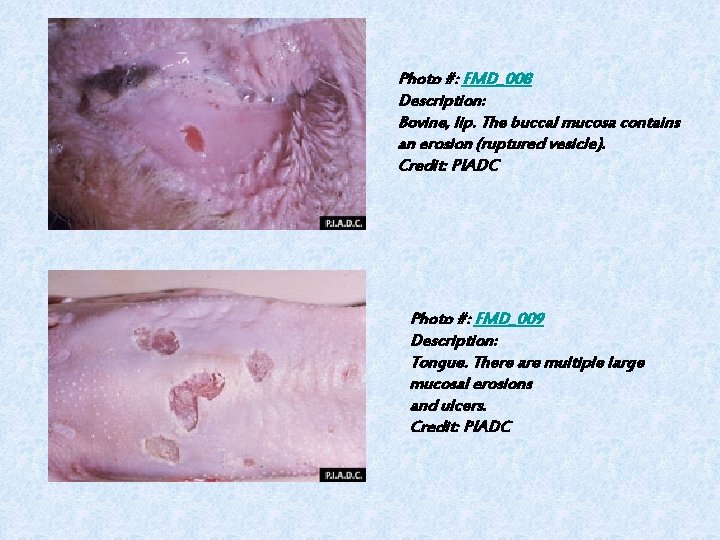
Photo #: FMD_008 Description: Bovine, lip. The buccal mucosa contains an erosion (ruptured vesicle). Credit: PIADC Photo #: FMD_009 Description: Tongue. There are multiple large mucosal erosions and ulcers. Credit: PIADC

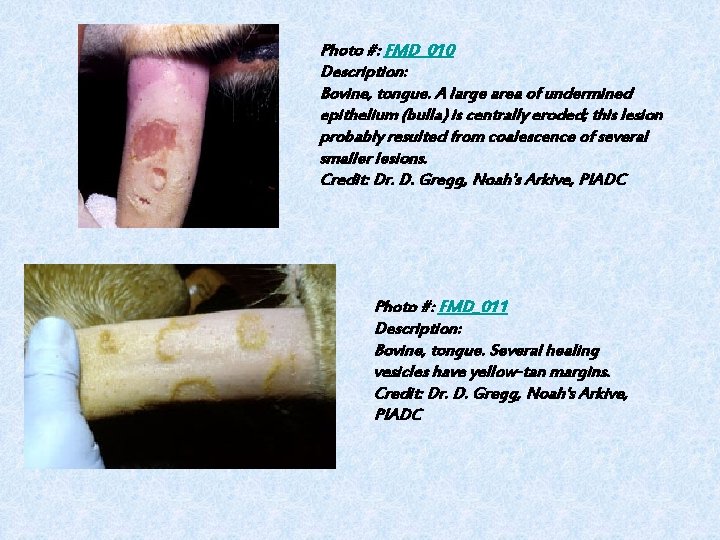
Photo #: FMD_010 Description: Bovine, tongue. A large area of undermined epithelium (bulla) is centrally eroded; this lesion probably resulted from coalescence of several smaller lesions. Credit: Dr. D. Gregg, Noah's Arkive, PIADC Photo #: FMD_011 Description: Bovine, tongue. Several healing vesicles have yellow-tan margins. Credit: Dr. D. Gregg, Noah's Arkive, PIADC

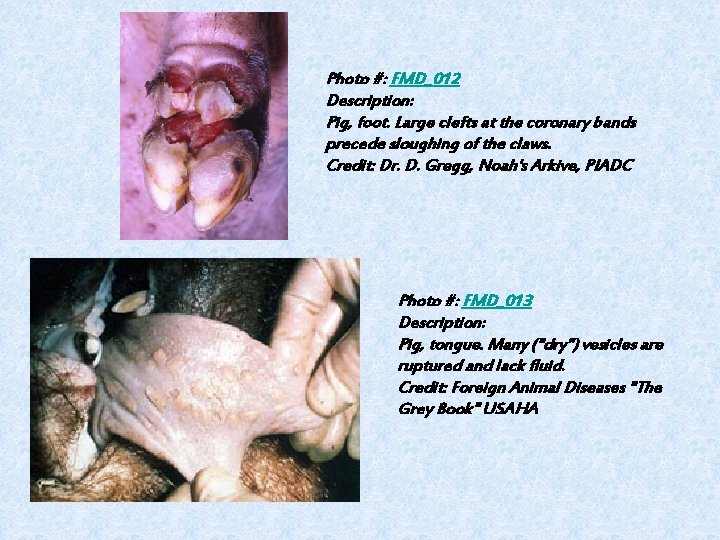
Photo #: FMD_012 Description: Pig, foot. Large clefts at the coronary bands precede sloughing of the claws. Credit: Dr. D. Gregg, Noah's Arkive, PIADC Photo #: FMD_013 Description: Pig, tongue. Many ("dry") vesicles are ruptured and lack fluid. Credit: Foreign Animal Diseases "The Grey Book" USAHA

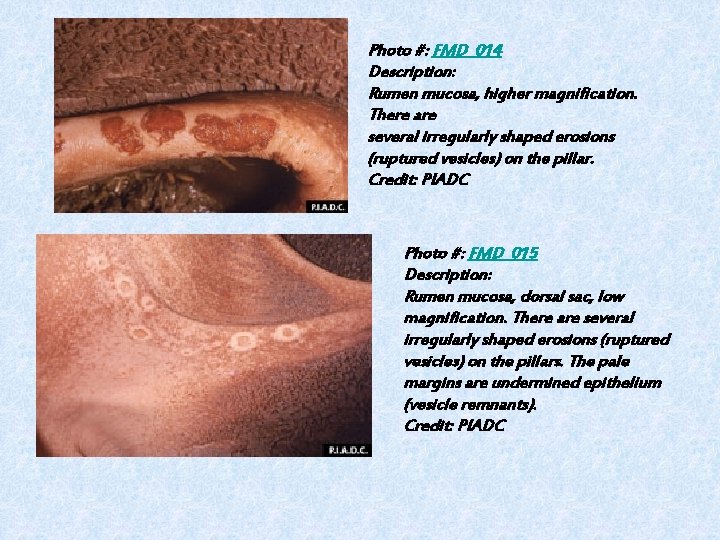
Photo #: FMD_014 Description: Rumen mucosa, higher magnification. There are several irregularly shaped erosions (ruptured vesicles) on the pillar. Credit: PIADC Photo #: FMD_015 Description: Rumen mucosa, dorsal sac, low magnification. There are several irregularly shaped erosions (ruptured vesicles) on the pillars. The pale margins are undermined epithelium (vesicle remnants). Credit: PIADC

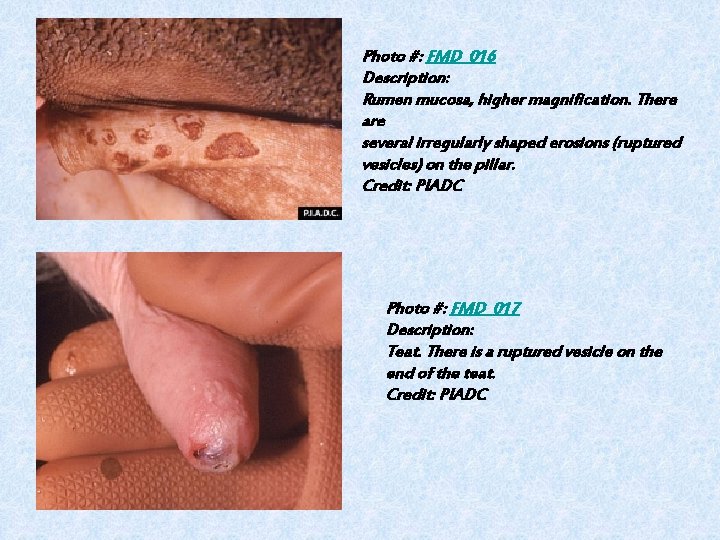
Photo #: FMD_016 Description: Rumen mucosa, higher magnification. There are several irregularly shaped erosions (ruptured vesicles) on the pillar. Credit: PIADC Photo #: FMD_017 Description: Teat. There is a ruptured vesicle on the end of the teat. Credit: PIADC

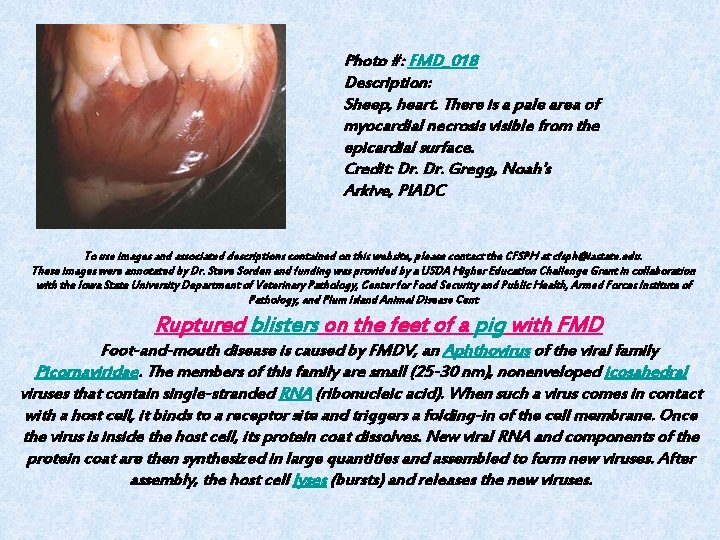
Photo #: FMD_018 Description: Sheep, heart. There is a pale area of myocardial necrosis visible from the epicardial surface. Credit: Dr. Gregg, Noah's Arkive, PIADC To use images and associated descriptions contained on this website, please contact the CFSPH at cfsph@iastate. edu. These images were annotated by Dr. Steve Sorden and funding was provided by a USDA Higher Education Challenge Grant in collaboration with the Iowa State University Department of Veterinary Pathology, Center for Food Security and Public Health, Armed Forces Institute of Pathology, and Plum Island Animal Disease Cent Ruptured blisters on the feet of a pig with FMD Foot-and-mouth disease is caused by FMDV, an Aphthovirus of the viral family Picornaviridae. The members of this family are small (25 -30 nm), nonenveloped icosahedral viruses that contain single-stranded RNA (ribonucleic acid). When such a virus comes in contact with a host cell, it binds to a receptor site and triggers a folding-in of the cell membrane. Once the virus is inside the host cell, its protein coat dissolves. New viral RNA and components of the protein coat are then synthesized in large quantities and assembled to form new viruses. After assembly, the host cell lyses (bursts) and releases the new viruses.

Ruptured oral vesicle in a cow with FMD can be transmitted in a number of ways including close contact animal to animal spread, long-distance aerosol spread and fomites or inanimate objects, typically fodder and motor vehicles. The clothes and skin of animal handlers such as farmers, standing water, and uncooked food scraps and feed supplements containing infected animal products can harbor the virus as well. Cows can also catch FMD from the semen of infected bulls. Control measures include quarantine and destruction of infected livestock, and export bans for meat and other animal products to countries not infected with the disease. When this shorthorn heifer in the United Kingdom became afflicted with foot-and-mouth disease, (a) she began to drool, and (b) virusfilled blisters developed in her mouth and at the junction where skin connects with the hoof walls

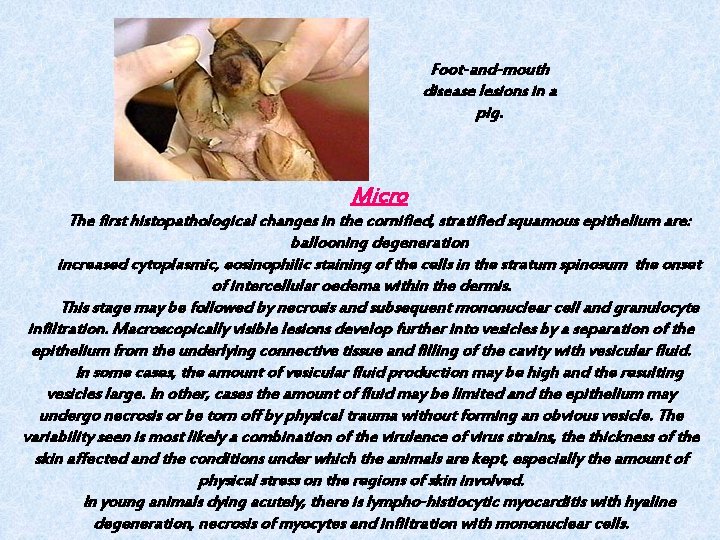
Foot-and-mouth disease lesions in a pig. Micro The first histopathological changes in the cornified, stratified squamous epithelium are: ballooning degeneration increased cytoplasmic, eosinophilic staining of the cells in the stratum spinosum the onset of intercellular oedema within the dermis. This stage may be followed by necrosis and subsequent mononuclear cell and granulocyte infiltration. Macroscopically visible lesions develop further into vesicles by a separation of the epithelium from the underlying connective tissue and filling of the cavity with vesicular fluid. In some cases, the amount of vesicular fluid production may be high and the resulting vesicles large. In other, cases the amount of fluid may be limited and the epithelium may undergo necrosis or be torn off by physical trauma without forming an obvious vesicle. The variability seen is most likely a combination of the virulence of virus strains, the thickness of the skin affected and the conditions under which the animals are kept, especially the amount of physical stress on the regions of skin involved. In young animals dying acutely, there is lympho-histiocytic myocarditis with hyaline degeneration, necrosis of myocytes and infiltration with mononuclear cells.

It is not possible to diagnose FMD based solely on the histologic appearance of the disease, as all vesicular diseases have similar histopathologic characteristics. There is intra- and intercellular edema in large rafts of cells within the stratum spongiosum. Once the vesicles have ruptured, the histology of vesicular diseases is very similar to many erosive and ulcerative diseases. Foot-and-mouth disease virus does not produce any observable viral inclusions Themortality in young animals, in particular lambs andpiglets, may be due to acute myocarditis. Myocarditis is considered a fatal form of FMD that occurs without vesiculation in young animals. 2, 4 The residual lesions in older cattle qualify as myocarditis with a significant inflammatory response. 39 However, the significance of acute myocarditis in the spread of FMDV has not been studied in detail. 2 The virus can usually be isolated from myocardia in areas containing necrotic myocytes that are infiltrated with mononuclear cells. 13

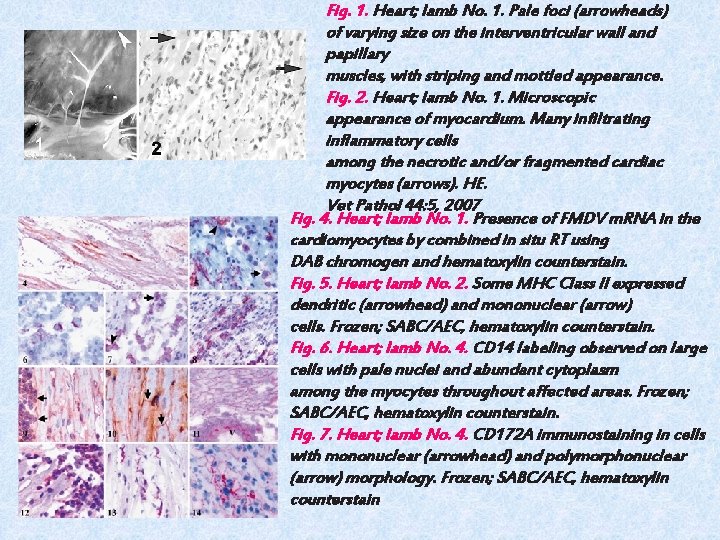
Fig. 1. Heart; lamb No. 1. Pale foci (arrowheads) of varying size on the interventricular wall and papillary muscles, with striping and mottled appearance. Fig. 2. Heart; lamb No. 1. Microscopic appearance of myocardium. Many infiltrating inflammatory cells among the necrotic and/or fragmented cardiac myocytes (arrows). HE. Vet Pathol 44: 5, 2007 Fig. 4. Heart; lamb No. 1. Presence of FMDV m. RNA in the cardiomyocytes by combined in situ RT using DAB chromogen and hematoxylin counterstain. Fig. 5. Heart; lamb No. 2. Some MHC Class II expressed dendritic (arrowhead) and mononuclear (arrow) cells. Frozen; SABC/AEC, hematoxylin counterstain. Fig. 6. Heart; lamb No. 4. CD 14 labeling observed on large cells with pale nuclei and abundant cytoplasm among the myocytes throughout affected areas. Frozen; SABC/AEC, hematoxylin counterstain. Fig. 7. Heart; lamb No. 4. CD 172 A immunostaining in cells with mononuclear (arrowhead) and polymorphonuclear (arrow) morphology. Frozen; SABC/AEC, hematoxylin counterstain

Pathological findings The 9 dead lambs subjected to examination had no clinical signs. However, all 9 lambs had myocardial lesions. The myocardial lesions had grayish or yellowish foci with irregular patterns ranging in size from pinpoint to 2 cm in diameter. These were seen mainly in the interventricular septum, but sometimes in the right ventricular free wall and in the papillary muscles (Fig. 1). No typical vesicular lesions were found in the mouths or on the feet. In some lambs, similar lesions were also seen on their epicardia. In the flock, however, some adult animals exhibited clinical signs of typical FMD, characterized by oral lesions, salivation, and mild lameness. Microscopic examination of heart tissues revealed inflammatory infiltration, hyaline degeneration, necrosis of sheets of myocytes with pyknotic nuclei, and occasionally fragmentation of myocytes. Myocardial degeneration to necrosis varied from random and multifocal to confluent in all lambs infected with FMDV. Cellular infiltration and hyaline degeneration did not show any predilection for specific sites in the myocardium. However, endocardial and epicardial sites were more affected in some lambs. Cellular infiltration was composed of mononuclear cells: mainly lymphocytes; macrophages; and, to a lesser extent, plasma cells. In addition, many neutrophils were seen at the periphery of the areas of necrosis and degeneration (Fig. 2).

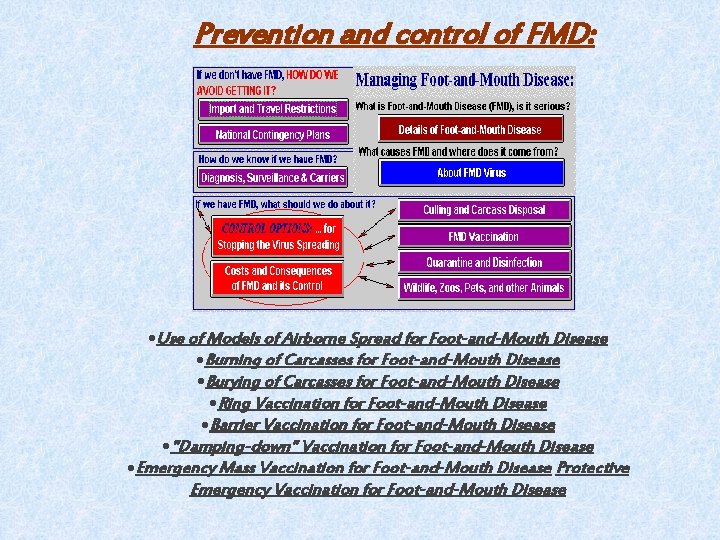
Prevention and control of FMD: Use of Models of Airborne Spread for Foot-and-Mouth Disease Burning of Carcasses for Foot-and-Mouth Disease Burying of Carcasses for Foot-and-Mouth Disease Ring Vaccination for Foot-and-Mouth Disease Barrier Vaccination for Foot-and-Mouth Disease "Damping-down" Vaccination for Foot-and-Mouth Disease Emergency Mass Vaccination for Foot-and-Mouth Disease Protective Emergency Vaccination for Foot-and-Mouth Disease

Control in an outbreak requires rapid and effective action. The USDA maintains emergency disease guidelines for all foreign animal diseases, including FMD. 13 In an outbreak, the amount of economic damage and the number of animals that will have to be destroyed are directly proportional to the length of time the disease is present prior to being accurately diagnosed. All infected and in-contact animals must be isolated and destroyed, as they are producing, or about to produce, large amounts of virus aerosols. 14 Monitoring through serosurveillance must be started immediately. Movement of humans, equipment, all materials including garbage, and nonsusceptible animals must also be closely monitored, and materials and equipment disinfected regularly. It is crucial to spray tires and the undersides of all vehicles leaving an infected area. Effective disinfectants include 2% acetic acid (half-strength kitchen vinegar) or 5. 25% sodium hypochlorite (3 parts laundry bleach, 2 parts water). If the initial focus is rapidly identified before any significant spread has occurred, ring vaccination is employed to create a "firewall" to keep the virus within a localized zone. 15 Only killed-virus, serotype-specific vaccines are available. Immunity from vaccination lasts only 6 months. A vaccinated animal could be infected with a second serotype and become clinically ill or could be infected with the same serotype and experience no clinical illness, but might be an effective shedder of the virus. 16 Once the outbreak has been contained, vaccinated animals are usually euthani

Refrences: References -- refereed Brown C, Slenning B. Impact and risk of foreign animal diseases. J Am Vet Med Assoc. 1996; 208: 1038 -1040 House J, Mebus C. Foot-and-mouth disease. In: Buisch W, Hyde J, Mebus C, eds. Foreign Animal Diseases. 6 th ed. Richmond, Virginia: United States Animal Health Association; 1998: 213 -224. Knowles N, Davies P, Henry T, O'Donnell V, Pacheco J, Mason P. Emergence in Asia of foot-and-mouth disease viruses with altered host range: characterization of alterations in the 3 A protein. J Virol. 2001; 75: 1551 -1556. Brown C, Olander H, Meyer R. Pathogenesis of foot-and-mouth disease in swine, studied by in-situ hybridization. J Comp Pathol. 1995; 113: 51 -58. Barnett P, Cox S. The role of small ruminants in the epidemiology and transmission of foot-and-mouth disease. Vet J. 1999; 158: 6 -13. Bergmann I, Malirat V, Auge de Mello P, Gomes I. Detection of foot-and-mouth disease viral sequences in various fluids and tissues during persistence of the virus in cattle. Am J Vet Res. 1996; 57: 134 -137. Sellers RF, Herniman KA, Mann JA. Transfer of foot-and-mouth disease virus in the nose of man from infected to non-infected animals. Vet Rec. 1971; 89: 447 -9. Ferguson N, Donnelly C, Anderson R. The foot-and-mouth epidemic in Great Britain: Pattern of spread and impact of interventions. Science. 2001; 292: 1155 -1160. Garland A. Vital elements for the successful control of foot-and-mouth disease by vaccination. Vaccine 1999; 17: 1760 -1766. Sellers R, Herniman K, Gumm I. The airborne dispersal of foot-and-mouth disease virus from vaccinated and recovered pigs, cattle and sheep after exposure to infection. Res Vet Sci. 1977; 23: 70 -75. References -- non refereed Brown C. Economic considerations of agricultural diseases. In: Frazier T, Richardson D, eds. Food and agricultural security: guarding against natural threats and terrorist attacks affecting health, national food supplies, and agricultural economics. Proceedings of an international conference. Washington DC, USA. Ann NY Acad Sci. 1999; 894: 92 -94. Rueckert R. Picornaviridae: The viruses and their replication. In: Fields B, Knipe D, Howley P, et al, eds. Field's Virology. 3 rd ed. Philadelphia: Lippincott. Raven Publishers; 1996: 609 -782 8. Donaldson A, Kitching P, Barnett P. Foot-and-mouth disease. In: Manual of Standards for Diagnostic Tests and Vaccines. Paris, France: Office International des Epizooties; 1996: 47 -56. 9. Office International des Epizooties Press Release. OIE emergency meeting on foot and mouth disease in East Asia. http: //www. oie. int/eng/press/A_000622. htm. Accessed July 18, 2001. 10. Source of the outbreak. Department for Environment, Food &Rural Affairs, 1 May 2001. www. maff. gov. uk/animalh/diseases/fmd/about/current/source. asp. Accessed July 20, 2001. 13. Foot-and-Mouth Disease Emergency Disease Guidelines. Riverdale, Maryland: US Dept of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services; 2001. 1 Ahn JS, Konno A, Gebe JA, Aruffo A, Hamilton MJ, Park YH, Davis WC: Scavenger receptor cysteine-rich domains 9 and 11 of WC 1 are receptors for the. WC 1 counter receptor. J Leukoc Biol 72: 382– 390, 2002 2 Alexandersen S, Mowat N: Foot-and-mouth disease: host range and pathogenesis. Curr Top Microbiol
 Cairo university faculty of veterinary medicine
Cairo university faculty of veterinary medicine Faculty of veterinary medicine cairo university logo
Faculty of veterinary medicine cairo university logo Faculty of veterinary medicine cairo university
Faculty of veterinary medicine cairo university Vet to vet
Vet to vet Snv constantine
Snv constantine Albumin in urine
Albumin in urine Webpath
Webpath Izharın hükmü
Izharın hükmü Le meridien cairo airport wedding packages
Le meridien cairo airport wedding packages Victor cairo arcelormittal
Victor cairo arcelormittal Pseudoconstipação do lactente
Pseudoconstipação do lactente Nile river absolute location
Nile river absolute location Patetta cairo montenotte
Patetta cairo montenotte Devolution ap human geography definition
Devolution ap human geography definition Mitchell cairo md
Mitchell cairo md Bishop sınıflaması
Bishop sınıflaması New cairo british international school
New cairo british international school Sabrina cairo
Sabrina cairo American university in cairo history
American university in cairo history Brt cairo
Brt cairo Tiempo cairo de dios
Tiempo cairo de dios Univ of texas arlington
Univ of texas arlington Mail univ ouargla
Mail univ ouargla Umbb fs
Umbb fs Univ valenciennes ent
Univ valenciennes ent Prodoc univ nantes
Prodoc univ nantes Pharmacie univ batna 2
Pharmacie univ batna 2 Faculté de médecine de constantine
Faculté de médecine de constantine Httpsfa
Httpsfa