ATOMS Atoms All material is made up of






- Slides: 14

ATOMS

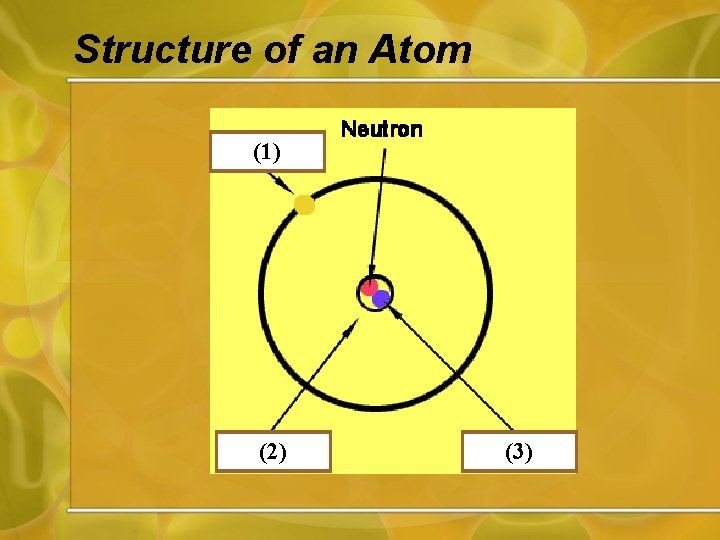
Atoms • All material is made up of tiny particles called atoms. • Atoms contain protons, neutrons and electrons.

Structure of an Atom (1) (2) (3)

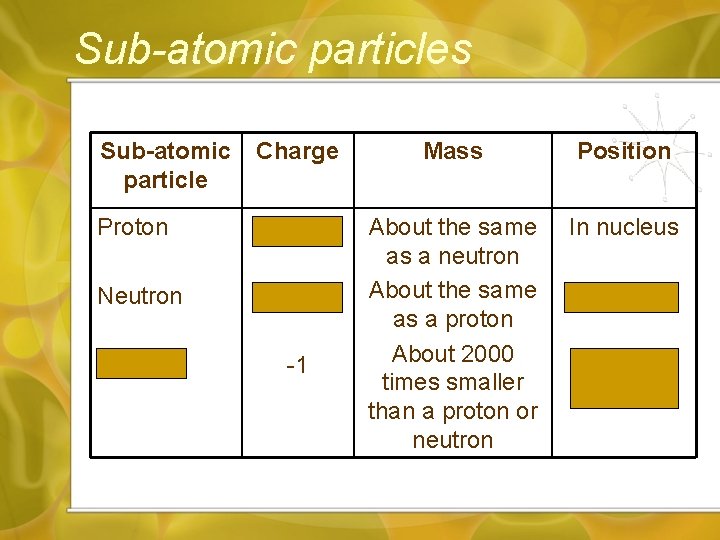
Sub-atomic particles Sub-atomic particle Charge Mass Position Proton +1 In nucleus Neutron 0 Electron -1 About the same as a neutron About the same as a proton About 2000 times smaller than a proton or neutron In nucleus Outside nucleus

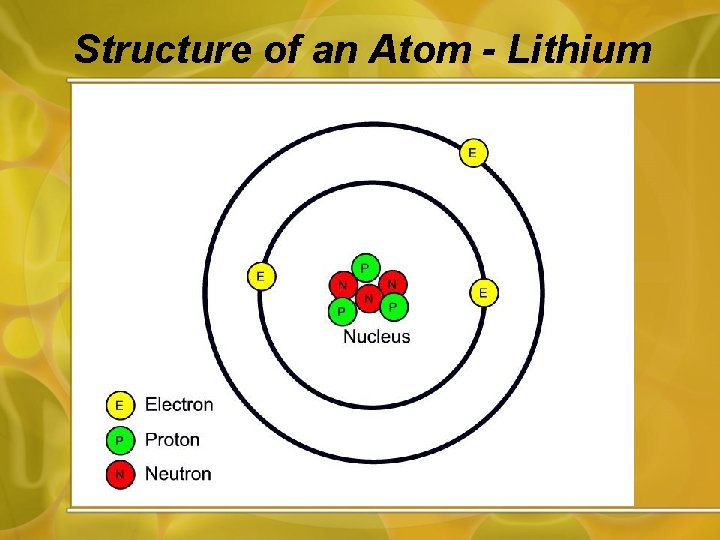
Structure of an Atom - Lithium

Structure of an Atom – Electron shells • Each electron shell can only hold a certain number of electrons. • The first electron shell can hold up to 2 electrons. • The second electron shell can hold up to 8 electrons. • The third electron shell can hold up to 8 electrons.

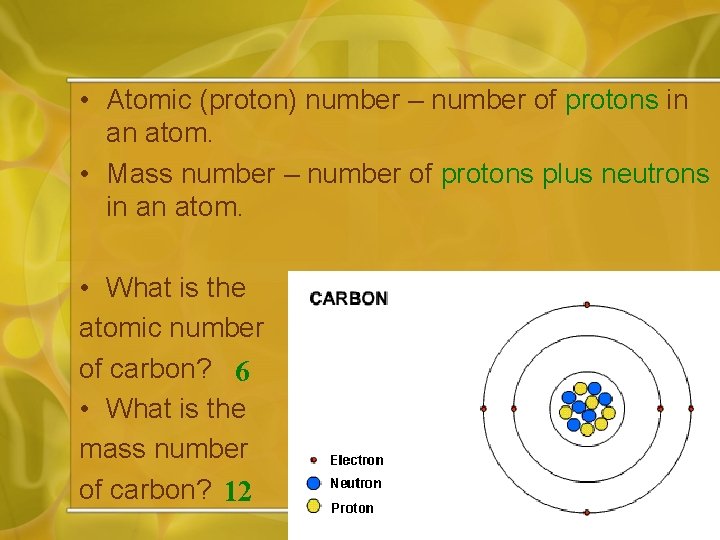
• Atomic (proton) number – number of protons in an atom. • Mass number – number of protons plus neutrons in an atom. • What is the atomic number of carbon? 6 • What is the mass number of carbon? 12

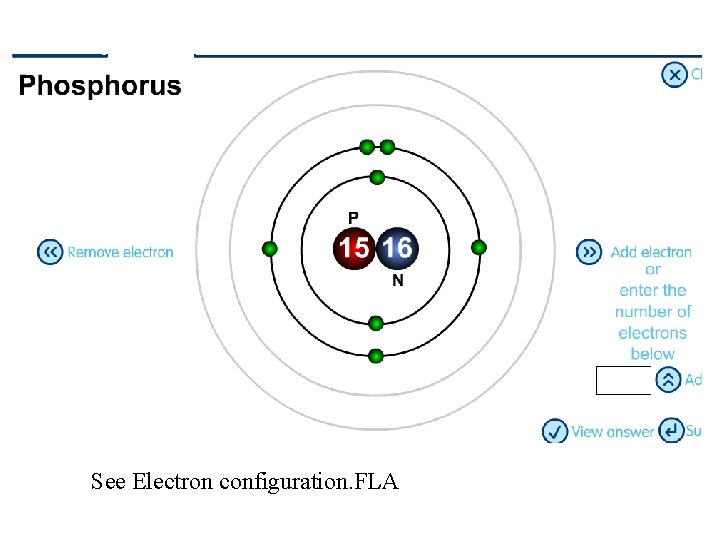
See Electron configuration. FLA



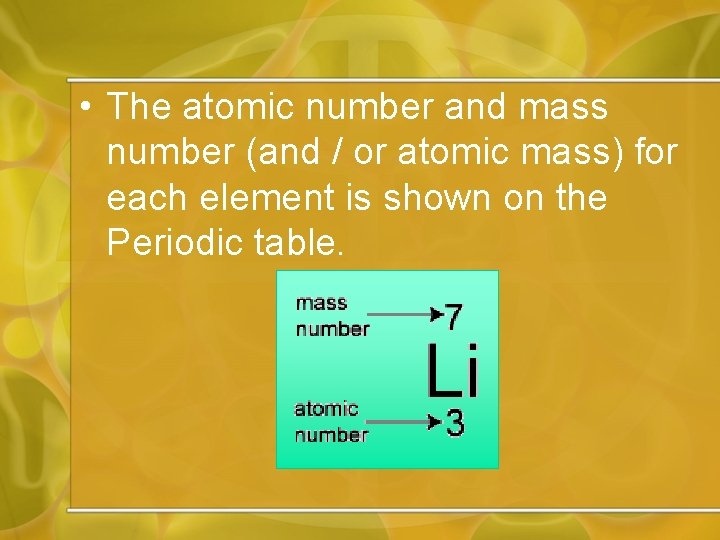
• The atomic number and mass number (and / or atomic mass) for each element is shown on the Periodic table.

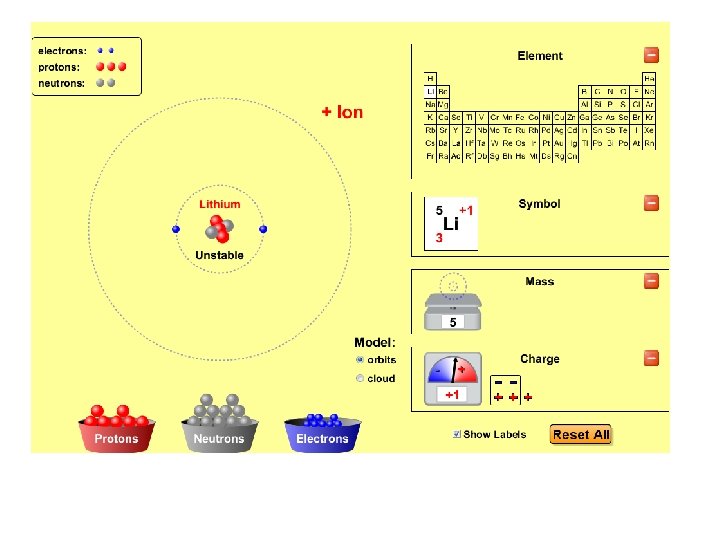
Ph. ET build-an-atom applet

Hank Helps Little Benny Bouncy Consistently Near Open Fire Nextdoor Nasty Mr Allen Said People Should Climb Around K Ca Harry Likes Beer But Could Nasty Mr Allen Said K Ca Not Often People Should He Find a. Ne Climb Around

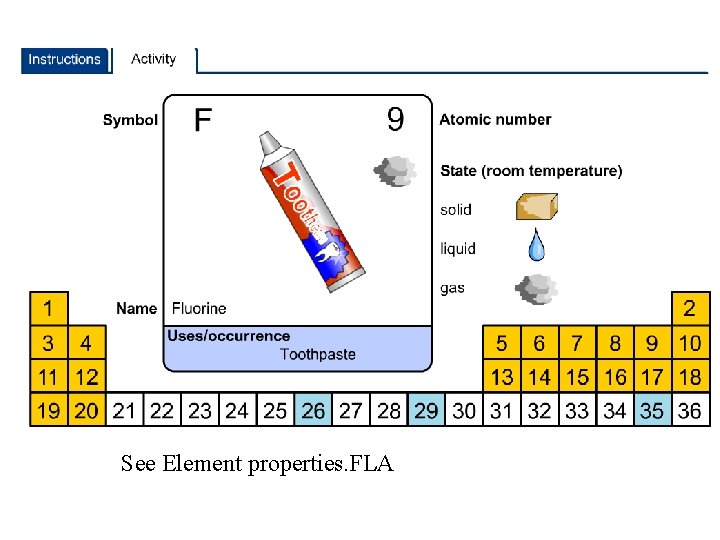
See Element properties. FLA
 Are all things made of atoms
Are all things made of atoms Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally Name a point that is collinear with the given points
Name a point that is collinear with the given points Bagian terkecil penyusun makhluk hidup adalah
Bagian terkecil penyusun makhluk hidup adalah Are cells made up of atoms
Are cells made up of atoms Flexible flat material made by interlacing yarns
Flexible flat material made by interlacing yarns How to play sahunay
How to play sahunay A long lasting paper like material made from reeds
A long lasting paper like material made from reeds What material is this made of
What material is this made of Two cylindrical resistors are made from the same material
Two cylindrical resistors are made from the same material Profile tolerance symbol
Profile tolerance symbol Variance analysis meaning
Variance analysis meaning Popular culture examples
Popular culture examples What is real culture
What is real culture Non material culture examples
Non material culture examples