APPILCMS Analysis of Acylglycerols ShengSuan Cai Luke Short
![Photoionization [A-m]+ + m S Fragmentation Energy [e. V] IP A+ IP Solvent (S) Photoionization [A-m]+ + m S Fragmentation Energy [e. V] IP A+ IP Solvent (S)](https://slidetodoc.com/presentation_image_h/0341f9ea47a3fe6ccd8442c1decc075d/image-2.jpg)

![EPA Methyl Ester (MW = 316) Mass Spectra APPI+ APCI+ [M+H]+ 9. 44 e EPA Methyl Ester (MW = 316) Mass Spectra APPI+ APCI+ [M+H]+ 9. 44 e](https://slidetodoc.com/presentation_image_h/0341f9ea47a3fe6ccd8442c1decc075d/image-8.jpg)
![Comparison of Detection Limits Monoarachidin 40 ESI Signal Nonlinear 120 Day 2 [M+Na]+ 20 Comparison of Detection Limits Monoarachidin 40 ESI Signal Nonlinear 120 Day 2 [M+Na]+ 20](https://slidetodoc.com/presentation_image_h/0341f9ea47a3fe6ccd8442c1decc075d/image-11.jpg)
![APPI Full Scan Mass Spectra of TAGs [M+H]+ [M-C 18: 0]+ [M+Na]+ SSS, C APPI Full Scan Mass Spectra of TAGs [M+H]+ [M-C 18: 0]+ [M+Na]+ SSS, C](https://slidetodoc.com/presentation_image_h/0341f9ea47a3fe6ccd8442c1decc075d/image-14.jpg)
- Slides: 20

APPI-LC/MS Analysis of Acylglycerols Sheng-Suan Cai, Luke Short, and Jack Syagen Technology, Inc. Jonathan Curtis Ocean Nutrition Canada 2/20/2021 Syagen Technology, Inc. 1411 Warner Avenue Tustin, CA 92780 www. syagen. com 2/20/2021
![Photoionization Am m S Fragmentation Energy e V IP A IP Solvent S Photoionization [A-m]+ + m S Fragmentation Energy [e. V] IP A+ IP Solvent (S)](https://slidetodoc.com/presentation_image_h/0341f9ea47a3fe6ccd8442c1decc075d/image-2.jpg)
Photoionization [A-m]+ + m S Fragmentation Energy [e. V] IP A+ IP Solvent (S) Analyte (A) Benefits of Photoionization Ionizes wide range of compounds (e. g. , non-polars, electronegative cpds, etc. ) Predominantly parent ion signal Minimum fragmentation Minimum solvent signal Minimum ion suppression Signal linear with concentration

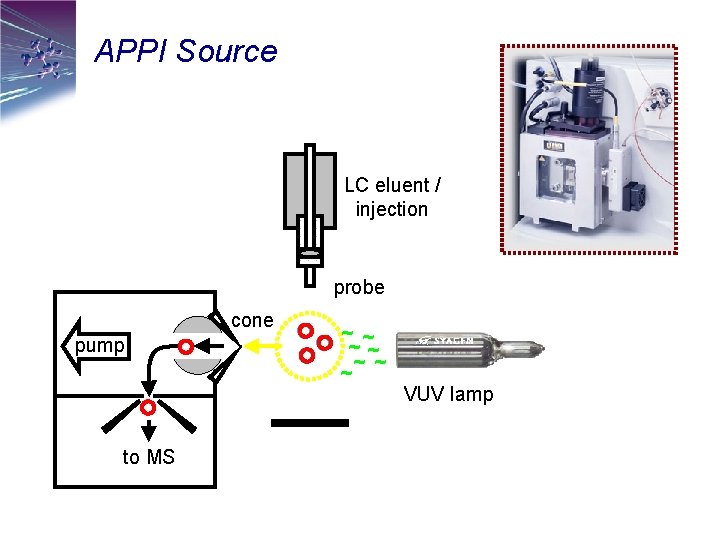
APPI Source LC eluent / injection probe cone pump to MS ~ ~ ~ ~ VUV lamp

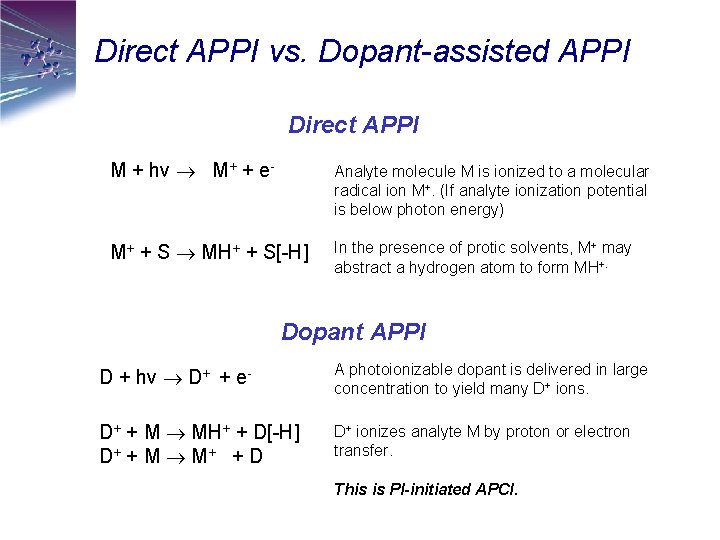
Direct APPI vs. Dopant-assisted APPI Direct APPI M + hv M+ + e- Analyte molecule M is ionized to a molecular radical ion M+. (If analyte ionization potential is below photon energy) M+ + S MH+ + S[-H] In the presence of protic solvents, M+ may abstract a hydrogen atom to form MH+. Dopant APPI D + hv D+ + e- A photoionizable dopant is delivered in large concentration to yield many D+ ions. D+ + M MH+ + D[-H] D+ + M M + + D D+ ionizes analyte M by proton or electron transfer. This is PI-initiated APCI.

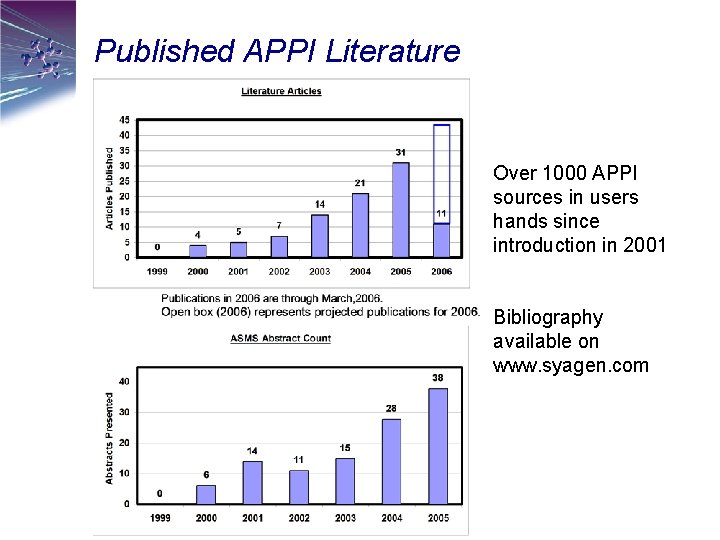
Published APPI Literature Over 1000 APPI sources in users hands since introduction in 2001 Bibliography available on www. syagen. com

Objectives ¿ Developed improved method relative to conventional methods ¿ GC or GC/MS requires tedious sample prep and analyte derivatization Conventional LC (i. e. , with UV or ELSD) lacks sensitivity and specificity Difficulties in analyzing nonpolar lipids by reversed phase LC/MS due to low solubility of analytes in reversed phase solvent systems (i. e. , Me. OH: H 2 O or CH 3 CN: H 2 O) Normal phase LC/MS may be better choice To investigate the advantage of using APPI over APCI and ESI for analysis of nonpolar lipids by comparing Mass spectra Dynamic linear range Sensitivity

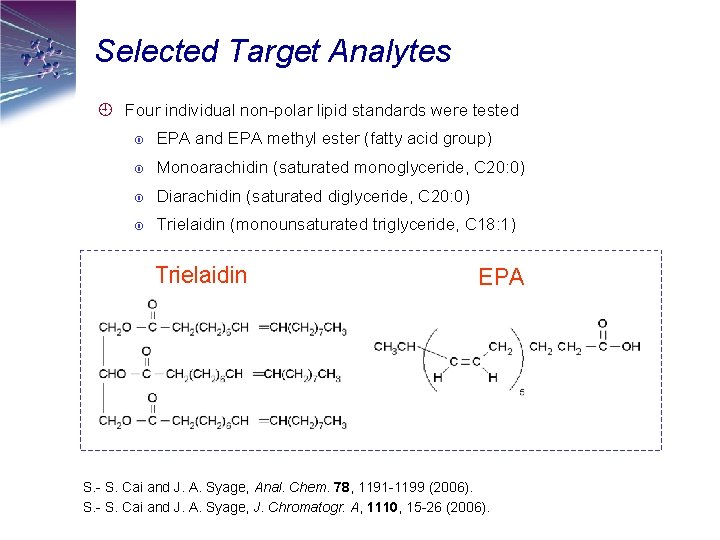
Selected Target Analytes ¿ Four individual non-polar lipid standards were tested EPA and EPA methyl ester (fatty acid group) Monoarachidin (saturated monoglyceride, C 20: 0) Diarachidin (saturated diglyceride, C 20: 0) Trielaidin (monounsaturated triglyceride, C 18: 1) Trielaidin EPA S. - S. Cai and J. A. Syage, Anal. Chem. 78, 1191 -1199 (2006). S. - S. Cai and J. A. Syage, J. Chromatogr. A, 1110, 15 -26 (2006).
![EPA Methyl Ester MW 316 Mass Spectra APPI APCI MH 9 44 e EPA Methyl Ester (MW = 316) Mass Spectra APPI+ APCI+ [M+H]+ 9. 44 e](https://slidetodoc.com/presentation_image_h/0341f9ea47a3fe6ccd8442c1decc075d/image-8.jpg)
EPA Methyl Ester (MW = 316) Mass Spectra APPI+ APCI+ [M+H]+ 9. 44 e 5 5. 99 e 5 ESI+ [M+H]+ ESI+ [M+Na]+ [M+H]+ 1. 71 e 5 [M+NH 4]+ 9. 36 e 5 [M+Na]+ APPI and APCI mobile phase was hexane, ESI mobile phase was 1: 1 isooctane/IPA without or with 10 m. M ammonium formate

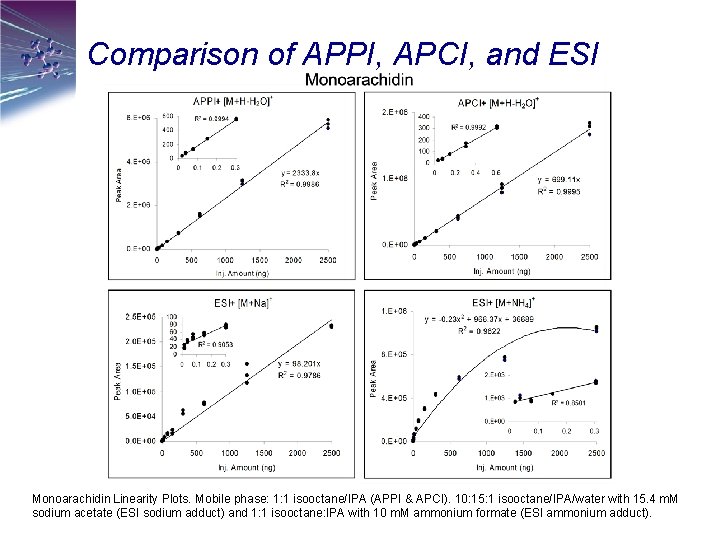
Comparison of APPI, APCI, and ESI Monoarachidin Linearity Plots. Mobile phase: 1: 1 isooctane/IPA (APPI & APCI). 10: 15: 1 isooctane/IPA/water with 15. 4 m. M sodium acetate (ESI sodium adduct) and 1: 1 isooctane: IPA with 10 m. M ammonium formate (ESI ammonium adduct).

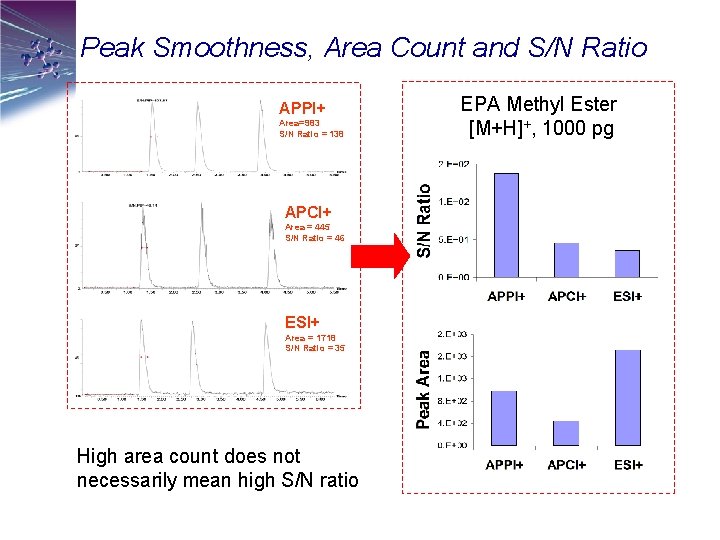
Peak Smoothness, Area Count and S/N Ratio APPI+ Area=983 S/N Ratio = 138 APCI+ Area = 445 S/N Ratio = 46 ESI+ Area = 1718 S/N Ratio = 35 High area count does not necessarily mean high S/N ratio EPA Methyl Ester [M+H]+, 1000 pg
![Comparison of Detection Limits Monoarachidin 40 ESI Signal Nonlinear 120 Day 2 MNa 20 Comparison of Detection Limits Monoarachidin 40 ESI Signal Nonlinear 120 Day 2 [M+Na]+ 20](https://slidetodoc.com/presentation_image_h/0341f9ea47a3fe6ccd8442c1decc075d/image-11.jpg)
Comparison of Detection Limits Monoarachidin 40 ESI Signal Nonlinear 120 Day 2 [M+Na]+ 20 Day 1 10 [M+NH 4]+ [M+Na]+ 0 APPI+ APCI+ ESI+ Diarachidin ESI Linear up to only 10 ng ESI+ DL (pg) 30 100 80 60 [M+NH 4]]++ [M+NH 4 40 20 0 APPI+ APCI+ ESI Linear up to only 5 ng Ø ESI [M+Na]+ signal unstable, Ø Na. OAc causes source fouling, Ø ESI [M+NH 4]+ poor linearity, nonlinear or extremely narrow linear range [M+NH 4]+

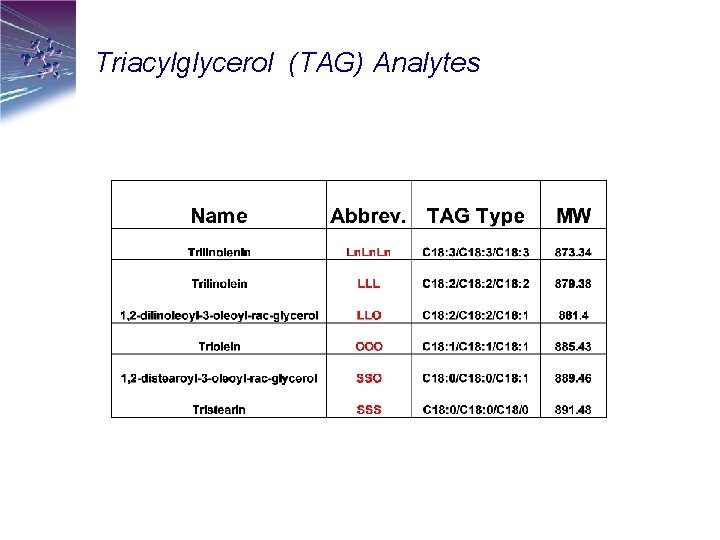
Triacylglycerol (TAG) Analytes

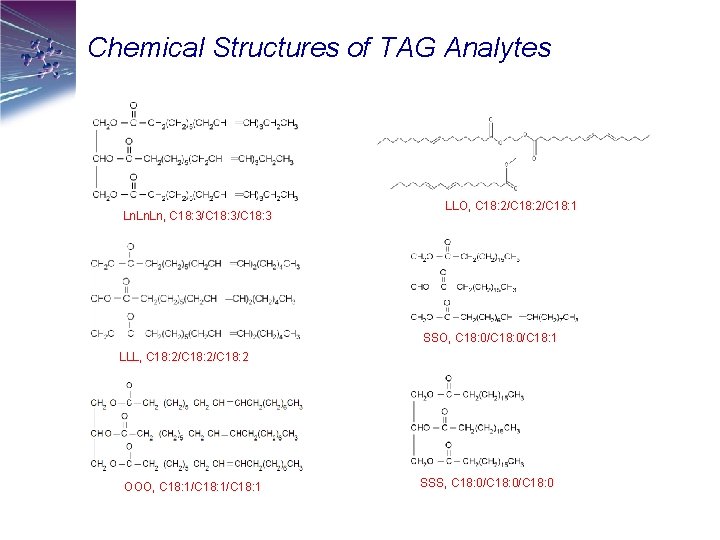
Chemical Structures of TAG Analytes Ln. Ln, C 18: 3/C 18: 3 LLO, C 18: 2/C 18: 1 SSO, C 18: 0/C 18: 1 LLL, C 18: 2/C 18: 2 OOO, C 18: 1/C 18: 1 SSS, C 18: 0/C 18: 0
![APPI Full Scan Mass Spectra of TAGs MH MC 18 0 MNa SSS C APPI Full Scan Mass Spectra of TAGs [M+H]+ [M-C 18: 0]+ [M+Na]+ SSS, C](https://slidetodoc.com/presentation_image_h/0341f9ea47a3fe6ccd8442c1decc075d/image-14.jpg)
APPI Full Scan Mass Spectra of TAGs [M+H]+ [M-C 18: 0]+ [M+Na]+ SSS, C 18: 0/C 18: 0 [M-C 18: 2]+ [M-C 18: 1]+ LLO, C 18: 2/C 18: 1 [M-C 18: 1]+ [M+H]+ [M-C 18: 0]+ [M+Na]+ SSO, C 18: 0/C 18: 1 [M-C 18: 2]+ LLL, C 18: 2/C 18: 2 [M-C 18: 1]+ [M+H]+ [M+Na]+ [M+H]+ OOO, C 18: 1/C 18: 1 [M-C 18: 3]+ Ln. Ln, C 18: 3/C 18: 3 As degree of unsaturation increases, [M+H]+ intensity increases

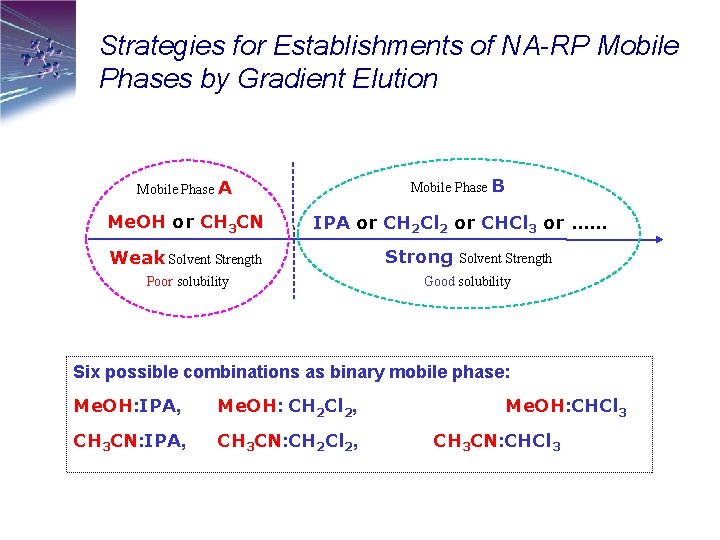
Strategies for Establishments of NA-RP Mobile Phases by Gradient Elution Mobile Phase A Mobile Phase B Me. OH or CH 3 CN IPA or CH 2 Cl 2 or CHCl 3 or …… Weak Solvent Strength Poor solubility Strong Solvent Strength Good solubility Six possible combinations as binary mobile phase: Me. OH: IPA, Me. OH: CH 2 Cl 2, CH 3 CN: IPA, CH 3 CN: CH 2 Cl 2, Me. OH: CHCl 3 CH 3 CN: CHCl 3

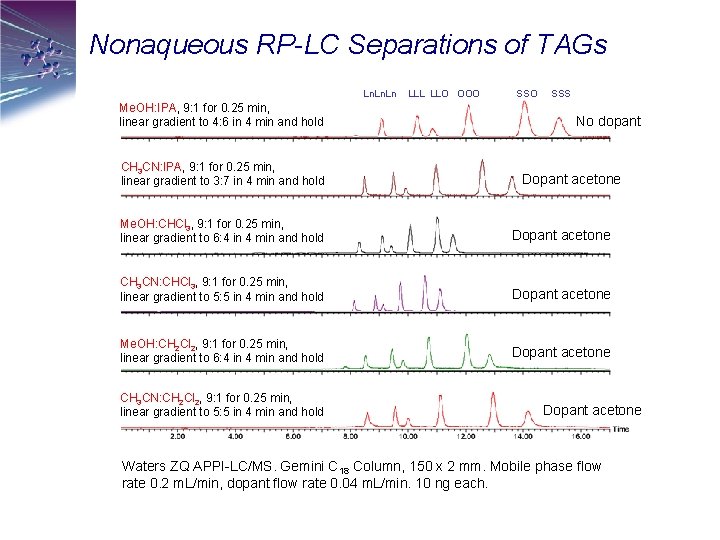
Nonaqueous RP-LC Separations of TAGs Ln. Ln Me. OH: IPA, 9: 1 for 0. 25 min, linear gradient to 4: 6 in 4 min and hold CH 3 CN: IPA, 9: 1 for 0. 25 min, linear gradient to 3: 7 in 4 min and hold LLL LLO OOO SSS No dopant Dopant acetone Me. OH: CHCl 3, 9: 1 for 0. 25 min, linear gradient to 6: 4 in 4 min and hold Dopant acetone CH 3 CN: CHCl 3, 9: 1 for 0. 25 min, linear gradient to 5: 5 in 4 min and hold Dopant acetone Me. OH: CH 2 Cl 2, 9: 1 for 0. 25 min, linear gradient to 6: 4 in 4 min and hold Dopant acetone CH 3 CN: CH 2 Cl 2, 9: 1 for 0. 25 min, linear gradient to 5: 5 in 4 min and hold Dopant acetone Waters ZQ APPI-LC/MS. Gemini C 18 Column, 150 x 2 mm. Mobile phase flow rate 0. 2 m. L/min, dopant flow rate 0. 04 m. L/min. 10 ng each.

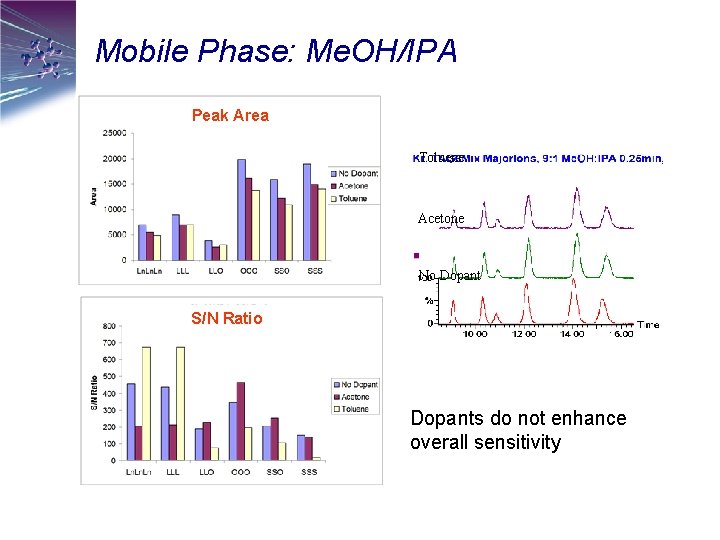
Mobile Phase: Me. OH/IPA Peak Area Toluene Acetone No Dopant S/N Ratio Dopants do not enhance overall sensitivity

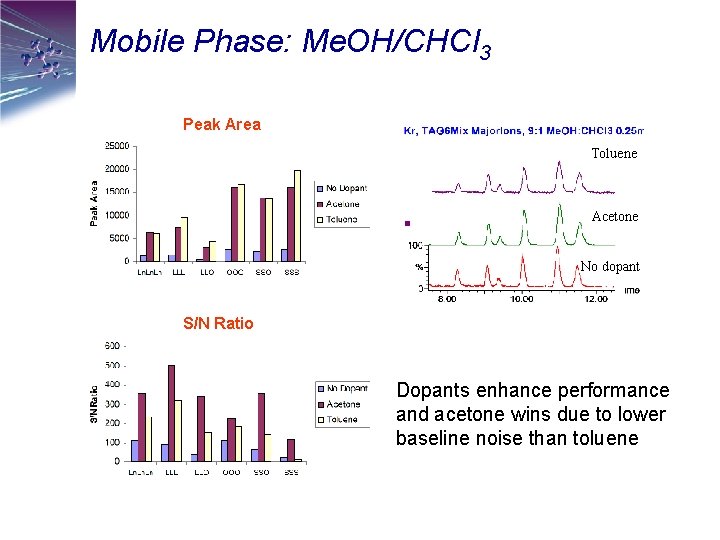
Mobile Phase: Me. OH/CHCl 3 Peak Area Toluene Acetone No dopant S/N Ratio Dopants enhance performance and acetone wins due to lower baseline noise than toluene

Summary and Conclusions ¿ Triacylglycerols in free acid and methyl ester forms in standards and in fish oils were studied by LC/MS using APPI, APCI, and ESI ¿ APPI and APCI offer comparable linear range (i. e. , 4 -5 decades) APPI is 2 -4 x more sensitive than APCI and much more sensitive than ESI w/o mobile phase additives. ESI sensitivity dramatically enhanced by mobile phase modifiers, but at much reduced linear range. Flow injection LODs <10 pg, and overall on-column LODs are 25 – 200 pg for a wide range of solvent conditions Use “APPI-Friendly” solvents such as IPA or Me. OH for high sensitivity w/o dopants Use CH 3 CN or CHCl 3 for lower column backpressure and better resolution, but dopants needed Acetone outperforms toluene as a dopant by not increasing and sometimes even suppressing baseline noise We acknowledge partial funding from NIH

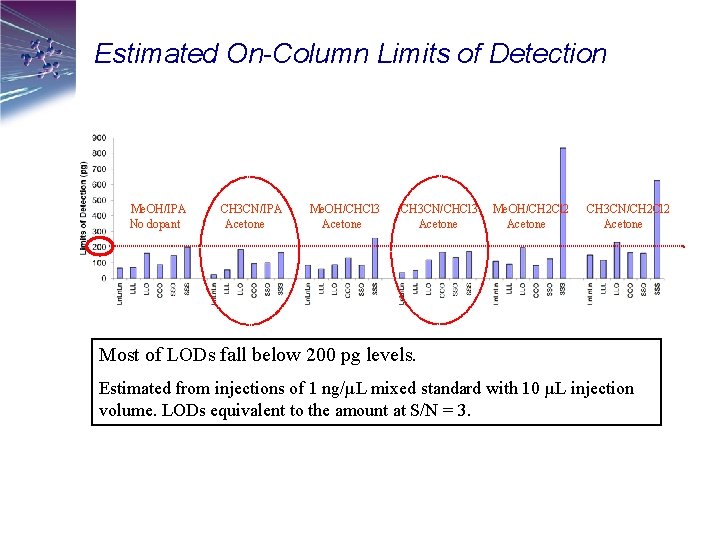
Estimated On-Column Limits of Detection Me. OH/IPA No dopant CH 3 CN/IPA Acetone Me. OH/CHCl 3 Acetone CH 3 CN/CHCl 3 Acetone Me. OH/CH 2 Cl 2 Acetone CH 3 CN/CH 2 Cl 2 Acetone Most of LODs fall below 200 pg levels. Estimated from injections of 1 ng/µL mixed standard with 10 µL injection volume. LODs equivalent to the amount at S/N = 3.
 Long and short
Long and short Modes of cai
Modes of cai Cai seniores milano
Cai seniores milano Hoa gì
Hoa gì Cai tutorial
Cai tutorial Contoh computer assisted instruction
Contoh computer assisted instruction Cai sezione di castelli te
Cai sezione di castelli te Mpl county cat
Mpl county cat Bọn em hai đứa cùng tên
Bọn em hai đứa cùng tên Hướng dẫn sử dụng case studio 2
Hướng dẫn sử dụng case studio 2 Sừng trâu
Sừng trâu Nếu thả một hòn đá nhỏ bên cạnh con rùa
Nếu thả một hòn đá nhỏ bên cạnh con rùa Integramias.caixa
Integramias.caixa Me cai del mundo galeano
Me cai del mundo galeano Cái cối tân
Cái cối tân Delia florea
Delia florea Cai guo qiang footprints of history
Cai guo qiang footprints of history Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Một thùng hàng có 1250 cái cốc và chén
Một thùng hàng có 1250 cái cốc và chén Lsm module
Lsm module Zhuozhen cai
Zhuozhen cai