Anesthetic Considerations for Pediatric Patients With Sickle Cell




















- Slides: 46

Anesthetic Considerations for Pediatric Patients With Sickle Cell Disease Andrew Infosino, MD UCSF Department of Anesthesia And Perioperative Care

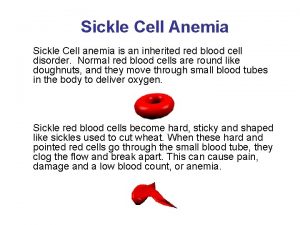
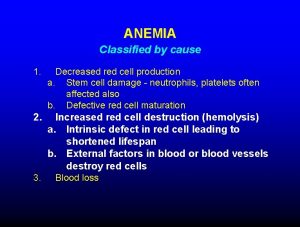
What is Sickle Cell Disease? Heterogenous group of inherited disorders of the β hemoglobin molecule. Clinical Manifestations include: • Chronic hemolytic anemia • Intermittent vaso-occclusive crises • Marked variability in severity from individual to individual • Average life expectancy in the developed world is 40 -60 years

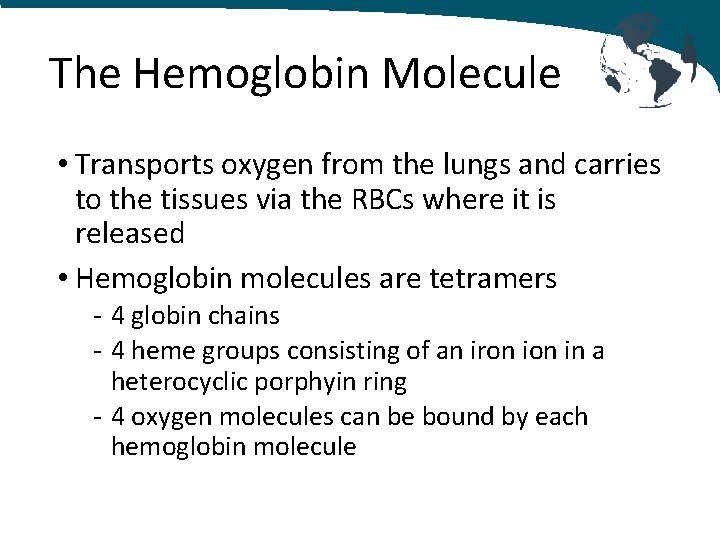
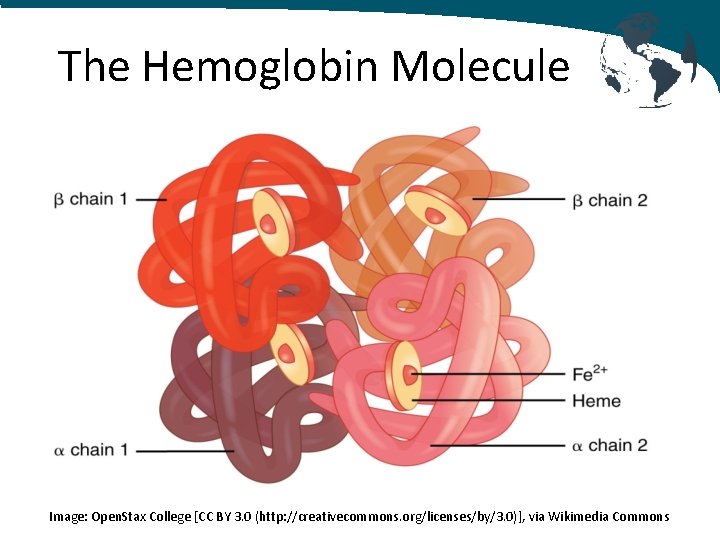
The Hemoglobin Molecule • Transports oxygen from the lungs and carries to the tissues via the RBCs where it is released • Hemoglobin molecules are tetramers - 4 globin chains - 4 heme groups consisting of an iron in a heterocyclic porphyin ring - 4 oxygen molecules can be bound by each hemoglobin molecule

The Hemoglobin Molecule Image: Open. Stax College [CC BY 3. 0 (http: //creativecommons. org/licenses/by/3. 0)], via Wikimedia Commons

Normal Hemoglobin in RBCs • Hemoglobin also carries carbon dioxide, but not at the iron binding positions, but in the globin chains • Hemoglobin also transports nitric oxide on specific thiol groups in the globin protein. Nitric oxide is released to peripheral tissues when oxygen is released leading to increased vasodilation

Normal Hemoglobin in RBCs Hemoglobin can also competitively bind other molecules such as carbon monoxide, cyanide, sulfur monoxide sulfide and hydrogen sulfide inhibiting oxygen binding and preventing delivery of oxygen to the tissues

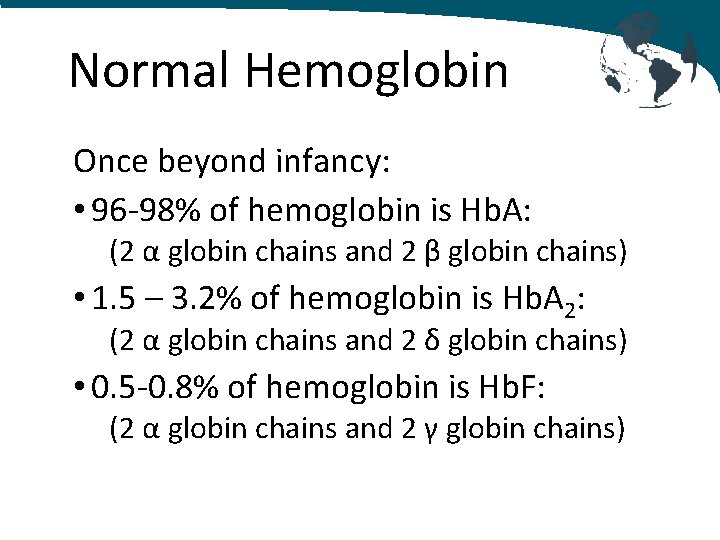
Normal Hemoglobin Once beyond infancy: • 96 -98% of hemoglobin is Hb. A: (2 α globin chains and 2 β globin chains) • 1. 5 – 3. 2% of hemoglobin is Hb. A 2: (2 α globin chains and 2 δ globin chains) • 0. 5 -0. 8% of hemoglobin is Hb. F: (2 α globin chains and 2 γ globin chains)

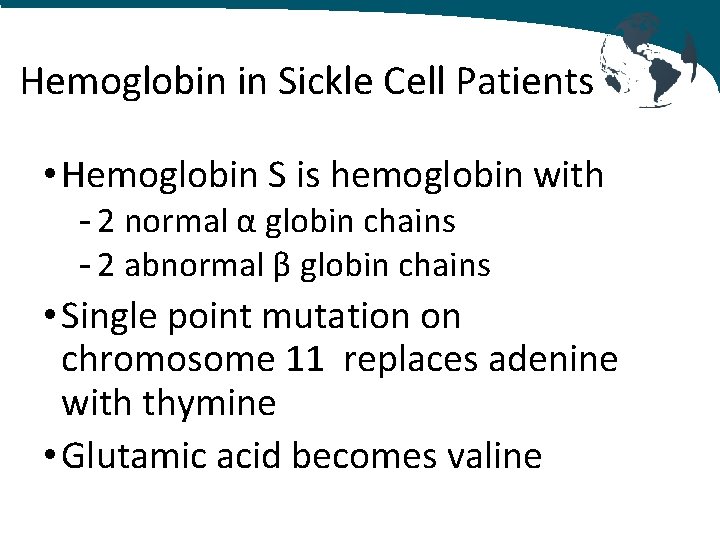
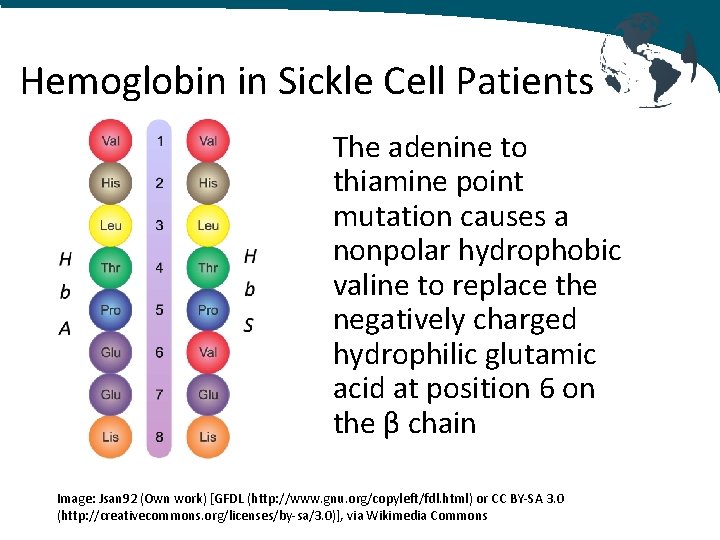
Hemoglobin in Sickle Cell Patients • Hemoglobin S is hemoglobin with - 2 normal α globin chains - 2 abnormal β globin chains • Single point mutation on chromosome 11 replaces adenine with thymine • Glutamic acid becomes valine

Hemoglobin in Sickle Cell Patients The adenine to thiamine point mutation causes a nonpolar hydrophobic valine to replace the negatively charged hydrophilic glutamic acid at position 6 on the β chain Image: Jsan 92 (Own work) [GFDL (http: //www. gnu. org/copyleft/fdl. html) or CC BY-SA 3. 0 (http: //creativecommons. org/licenses/by-sa/3. 0)], via Wikimedia Commons

Hemoglobin in Sickle Cell Patients • Loss of negative charge destabilizes the oxygenated hemoglobin resulting in accelerated denaturation and breakdown • Results in hemolytic anemia • RBC membranes exposed to oxidant potential of intracellular iron (normally iron is contained within hydrophobic globin pocket) • Damaged RBC membranes lead to RBC dehydration and increased RBC sickling

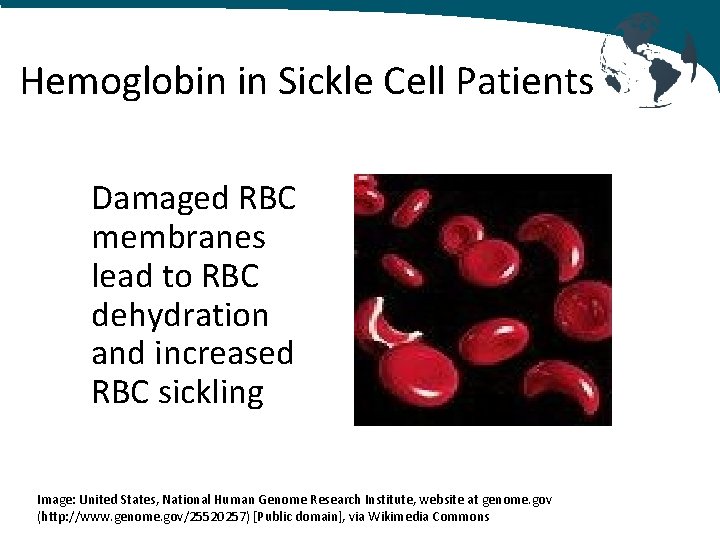
Hemoglobin in Sickle Cell Patients Damaged RBC membranes lead to RBC dehydration and increased RBC sickling Image: United States, National Human Genome Research Institute, website at genome. gov (http: //www. genome. gov/25520257) [Public domain], via Wikimedia Commons

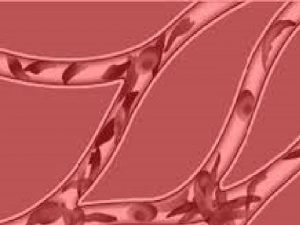
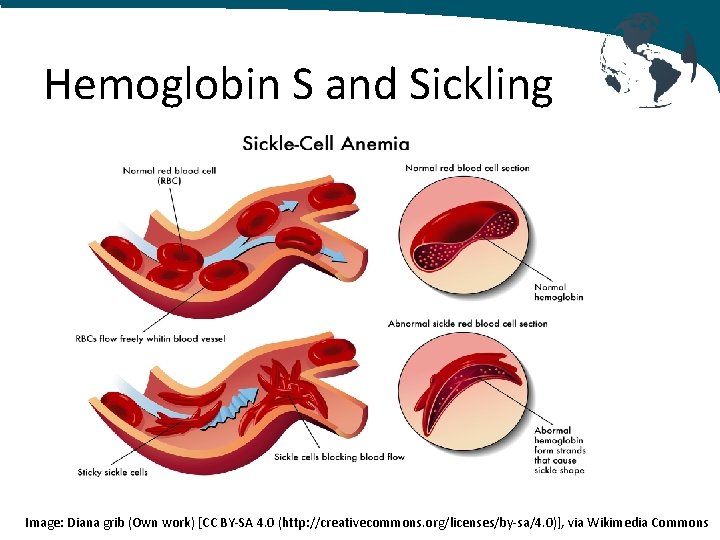
Hemoglobin S and Sickling • Conformational change during deoygenation allows a hydrophobic bond to form between βS-6 valine of one tetramer and the βS-85 phenylalanine and βS-88 leucine on an adjacent tetramer • Results in polymerization which distorts the shape of red blood cell • The “sickling” causes increased viscosity and occlusion in the microvasculature

Hemoglobin S and Sickling Image: Diana grib (Own work) [CC BY-SA 4. 0 (http: //creativecommons. org/licenses/by-sa/4. 0)], via Wikimedia Commons

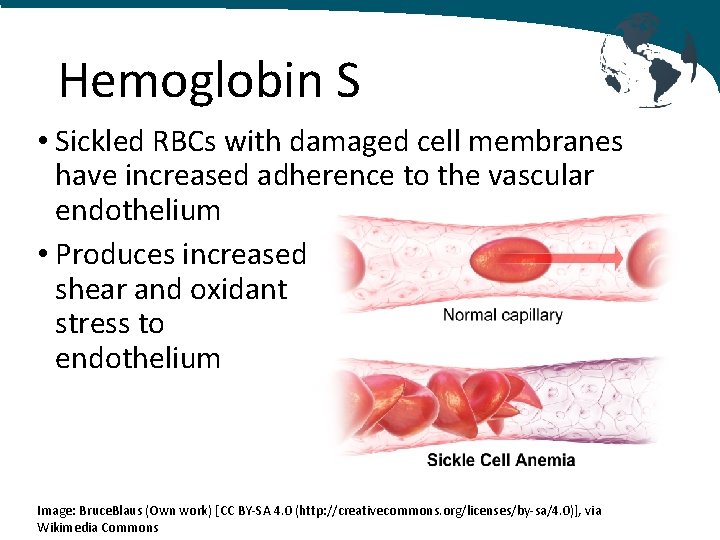
Hemoglobin S • Sickled RBCs with damaged cell membranes have increased adherence to the vascular endothelium • Produces increased shear and oxidant stress to endothelium Image: Bruce. Blaus (Own work) [CC BY-SA 4. 0 (http: //creativecommons. org/licenses/by-sa/4. 0)], via Wikimedia Commons

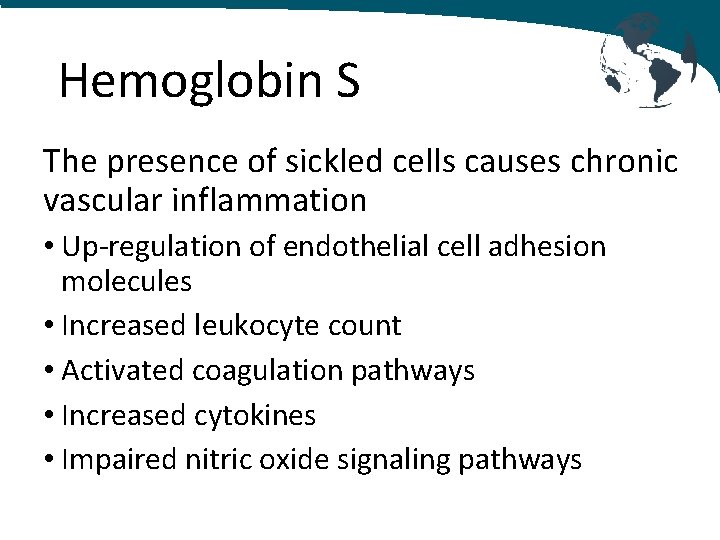
Hemoglobin S The presence of sickled cells causes chronic vascular inflammation • Up-regulation of endothelial cell adhesion molecules • Increased leukocyte count • Activated coagulation pathways • Increased cytokines • Impaired nitric oxide signaling pathways

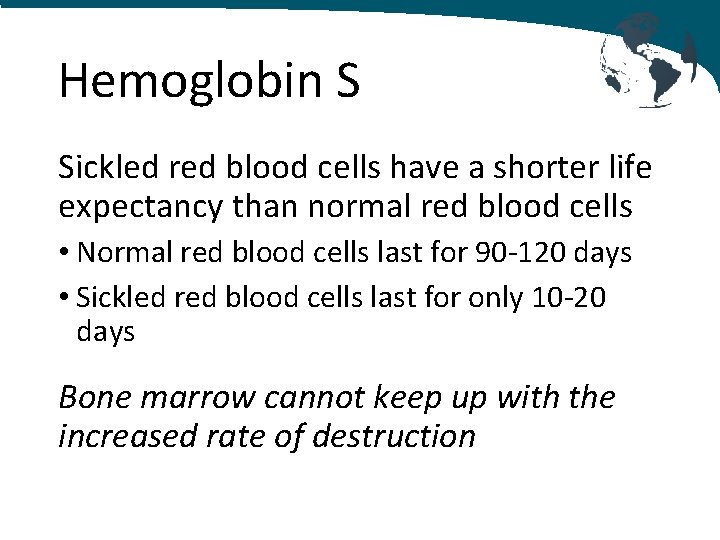
Hemoglobin S Sickled red blood cells have a shorter life expectancy than normal red blood cells • Normal red blood cells last for 90 -120 days • Sickled red blood cells last for only 10 -20 days Bone marrow cannot keep up with the increased rate of destruction

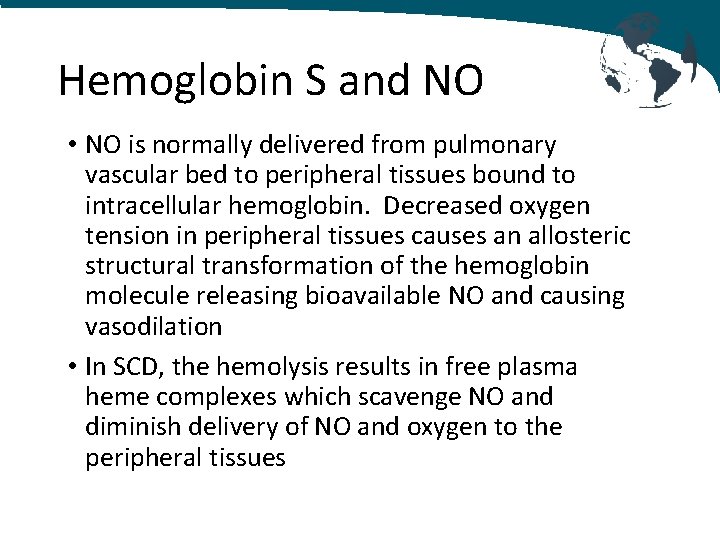
Hemoglobin S and NO • NO is normally delivered from pulmonary vascular bed to peripheral tissues bound to intracellular hemoglobin. Decreased oxygen tension in peripheral tissues causes an allosteric structural transformation of the hemoglobin molecule releasing bioavailable NO and causing vasodilation • In SCD, the hemolysis results in free plasma heme complexes which scavenge NO and diminish delivery of NO and oxygen to the peripheral tissues

Sickle Cell Disease Inheritance • Autosomal recessive inheritance pattern • Clinical disease occurs in patients homozygous for Hb. S gene: Hb. SS • A person who receives a gene for sickle cell disease from one parent and a normal gene from the other has a condition called "sickle cell trait"

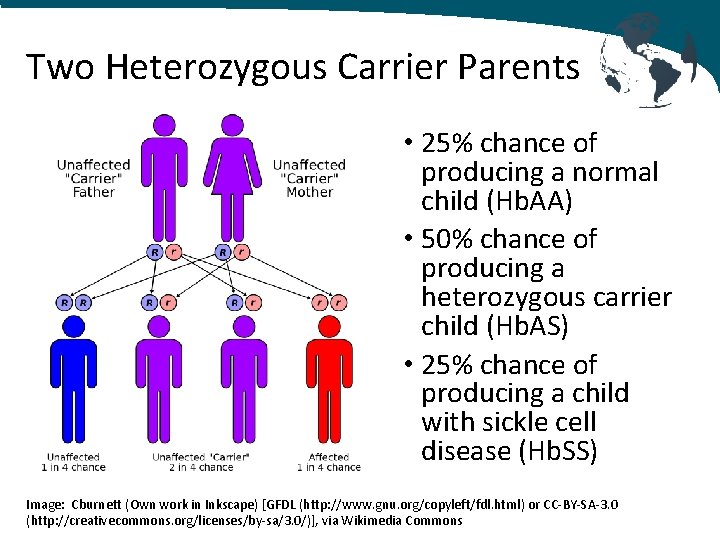
Two Heterozygous Carrier Parents • 25% chance of producing a normal child (Hb. AA) • 50% chance of producing a heterozygous carrier child (Hb. AS) • 25% chance of producing a child with sickle cell disease (Hb. SS) Image: Cburnett (Own work in Inkscape) [GFDL (http: //www. gnu. org/copyleft/fdl. html) or CC-BY-SA-3. 0 (http: //creativecommons. org/licenses/by-sa/3. 0/)], via Wikimedia Commons

Vaso-occlusive Crises Vaso-occlusive crises are intermittent, recurrent, acute episodes of severe pain. Triggers include: • Infection • Surgical stress • Hypoxia • Altitude

Acute Vaso-occlusive Crises Sickled red blood cells adhere to vascular endothelium and cause microvascular occlusion, ischemia, infarction and pain • Inflammation of endothelium with release of IL-1, IL-6 and TNF • Platelet activation and aggregation • Fibrin deposition on damaged endothelium • Up-regulation of cell adhesion molecules • Decreased production and increased scavenging of nitric oxide • Activation of coagulation system

Pathophysiology of Sickle Cell Disease • Fully oxygenated Hb. SS blood is more viscous than fully oxygenated Hb. AA blood at the same hematocrit (less deformable RBCs) • The viscosity of Hb. SS blood increases more rapidly with increasing hematocrit than Hb. AA blood - Hb. AA blood: An increase of Hct from 30% to 60% increases viscosity x 2 - Hb. SS blood: An increase of Hct from 30% to 60% increases viscosity x 50

Types of Acute Sickle Cell Crises • Acute chest syndrome • Cerebral Vascular Accident or Stroke • Ischemia/Pain • Priapism • Splenic sequestration crisis • Aplastic crisis • Hyper-hemolytic crisis

SCD: Central Nervous System • Transient ischemic attacks • Silent Infarcts • Cerebrovascular accidents including intra-cerebral hemorrhages • Seizures

SCD: Hematology • Hemolytic crises from accelerated RBC death • Aplastic crises with suppression of normal erythropoiesis which usually resolve in 7 -10 days. Transient ischemic attacks Associated with infections from EBV, parvovirus B 19, Pneumococci, Salmonella and Streptococci

SCD: Cardiovascular • Cardiomegaly from chronic anemia • Left ventricular hypertrophy and congestive heart failure • Right ventricular hypertrophy from chronic hypoxia

SCD: Respiratory System • Micro-infarctions resulting in progressive pulmonary fibrosis and restrictive lung disease • Acute chest syndrome: Combination of infarction, infection, pulmonary sequestration and fat embolism

SCD: Gastrointestinal System • Repeated splenic micro-infarcts render patient functionally asplenic • Acute cholecystitis from bile stones from hemolysis often require cholecystectomy • Acute abdominal pain from vasoocclusive crisis

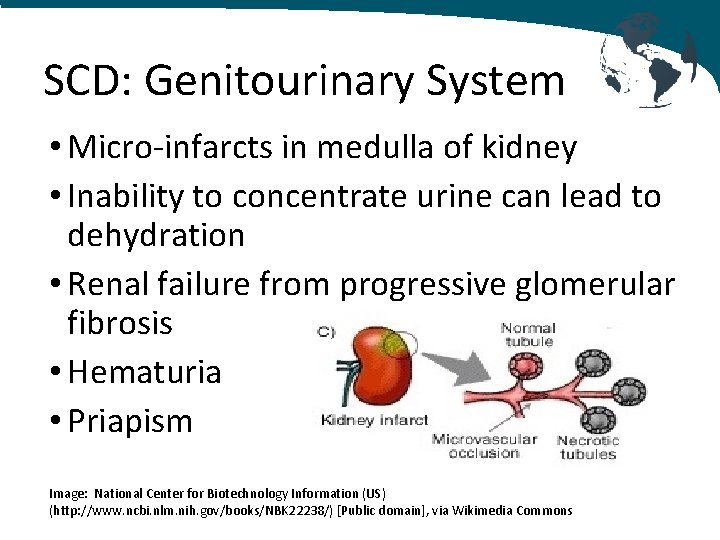
SCD: Genitourinary System • Micro-infarcts in medulla of kidney • Inability to concentrate urine can lead to dehydration • Renal failure from progressive glomerular fibrosis • Hematuria • Priapism Image: National Center for Biotechnology Information (US) (http: //www. ncbi. nlm. nih. gov/books/NBK 22238/) [Public domain], via Wikimedia Commons

SCD: Bones, Extremities & Growth • Increased bone marrow activity in bones starves the growth plates of nutrients resulting in decreased growth • Bone pain from vaso-occlusion in medulla of bones • Avascular necrosis of the femoral head • Increased incidence of osteomyelitis often due to Salmonella or Staphylococci

SCD: Presentation Striking diversity in presentation, progression of disease and severity of symptoms • About 30% of Hgb. SS patients have rapidly progressive disease with widespread vascular damage, organ failure and death • About 60% have less severe disease • About 10% are relatively asymptomatic

SCD: Presentation Diversity in presentation, progression of disease and severity of symptoms due to: • Increased expression of Hemoglobin F which ameliorates the disease • Haplotype variation • Coinheritance of α-thalassemia • Polymorphisms at cellular and physiologic levels

SCD: Presentation Increased production of Hemoglobin F: • Normal production of Hgb. F is 1% after infancy • African haplotypes express Hgb. F up to 15% • Asian haplotypes express Hgb. F from 8 -30%

Preoperative Anesthetic Management History and Physical Exam • Document the number and frequency of crises - No active infections - No acute pain crisis • Consult with patient’s hematologist - Baseline hematocrit - Baseline percentage of hemoglobin S - Preoperative transfusion recommendations

Preoperative Anesthetic Management Labs: Baseline Values should be obtained and compared to historical values • Hemoglobin/hematocrit • Hemoglobin electrophoresis for percentage of hemoglobin S

SCD: Historical Approaches to Preoperative Transfusion • Simple transfusion to raise the hemoglobin to >10 g/dl • Simple transfusion for high risk patients - History of frequent sickle crises - History of frequent acute chest syndrome crises - Complex surgical procedures with significant blood loss • Exchange transfusion to lower the Hb. S to <30% and raise the hemoglobin to >10 g/dl

SCD: Changes in Transfusion Practice HIV and Hepatitis transmission from blood transfusions prompted a reexamination of transfusion practices in sickle cell patients Image: Bruce. Blausen. com staff (2014). "Medical gallery of Blausen Medical 2014". Wiki. Journal of Medicine 1 (2). DOI: 10. 15347/wjm/2014. 010. ISSN 2002 -4436. (Own work) [CC BY 3. 0 (http: //creativecommons. org/licenses/by/3. 0)], via Wikimedia Commons

SCD: Current Preoperative Transfusion Practices • Exchange transfusions no longer recommended • Low risk patients and low risk procedures (short duration, minimal fluid shifts, no significant blood loss): Preoperative transfusion not recommended • High risk patients and/or high risk procedures: Simple transfusion with hemoglobin goal of between 9 – 11 g/dl (over-transfusion increases blood viscosity)

SCD: Preoperative Management • Schedule as first case of the day to minimize NPO time • Consider IV fluid bolus of 10 to 15 ml/kg after starting IV and before induction • Notify Blood Bank and send type and cross as soon as possible: May be more difficult to obtain compatible blood because of frequent transfusions and antibody formation

SCD: Goals of Intraoperative Management • Avoid hypotension • Avoid hypoxemia • Avoid hypothermia • Avoid hypovolemia

SCD: Intraoperative Management • Keep operating room and patient warm • Maintain Fi. O 2 of at least 40 - 50% • Use pneumatic compression devices to help minimize vascular stasis in the lower extremities • Antibiotic prophylaxis

SCD: Intraoperative Management • Regional anesthesia has been safely used in sickle cell patients and may be beneficial in patients with opioid tolerance • Tourniquets have been used in patients with SCD, but extremity should be carefully exsanguinated prior to inflation

Postoperative Management The majority of perioperative complications occur during the postoperative period and include: • Painful crises • Acute chest syndrome • Stroke

Postoperative Management Minimize postoperative complications with: • Postoperative oxygen therapy • Postoperative hydration • Postoperative pulse-oximetry monitoring • Postoperative pain control

Managing Chronic Pain in Sickle Cell Patients • Challenging intravascular access from multiple intravenous lines in the past • Pain: High tolerance to opioids • Opioid dependance Involve the Chronic Pain Team. Use multimodal pain management. Consider PCAs, epidurals, PCEAs, fentanyl patches, NSAIDs and acetaminophen

References: 1. Marchant WA, Walker I. Anaesthetic Management of the Child with Sickle Cell Disease. Paediatric Anaesthesia 2003; 13: 473 -489. 2. Firth PG, Head CA. Sickle Cell Disease and Anesthesia. Anesthesiology 2004; 101: 766 -785. 3. https: //en. wikipedia. org/wiki/Sickle-cell_disease.
 Life expectancy of sickle cell patients
Life expectancy of sickle cell patients Anesthetic, pungent, sweet
Anesthetic, pungent, sweet Vaporizer machine parts
Vaporizer machine parts Bioreactor considerations for animal cell culture
Bioreactor considerations for animal cell culture Sickle cell anemia symptoms
Sickle cell anemia symptoms Hemophilia karyotype picture
Hemophilia karyotype picture Sickle cell anaemia is
Sickle cell anaemia is Sickle cell karyotype
Sickle cell karyotype Gene therapy for sickle cell disease
Gene therapy for sickle cell disease Sickle cell pbs
Sickle cell pbs Anemia
Anemia Cri du chat chromosomal abnormalities
Cri du chat chromosomal abnormalities Sickle cell pain
Sickle cell pain Sickle blood cell
Sickle blood cell Sickle cell anemia dna sequence
Sickle cell anemia dna sequence Sickle cell anemia pedigree
Sickle cell anemia pedigree Old world flycatchers
Old world flycatchers Sickle cell hemoglobin structure
Sickle cell hemoglobin structure Is sickle cell anemia codominant
Is sickle cell anemia codominant Everardo cobos
Everardo cobos Sickle cell anemia symptoms
Sickle cell anemia symptoms Missense mutation in sickle cell anemia
Missense mutation in sickle cell anemia Sickle cell anemia
Sickle cell anemia Types of sickle cell disease
Types of sickle cell disease Persilangan thalasemia
Persilangan thalasemia Sickle cell hemoglobin structure
Sickle cell hemoglobin structure Nata sickle cell
Nata sickle cell Difference between sickle cell anaemia and thalassemia
Difference between sickle cell anaemia and thalassemia Sickle cell osteomyelitis
Sickle cell osteomyelitis Sickle cell genetics
Sickle cell genetics Pethidine in sickle cell
Pethidine in sickle cell Heterozygous type a blood
Heterozygous type a blood Genotypic ratio of monohybrid cross
Genotypic ratio of monohybrid cross Sickle cell punnett square
Sickle cell punnett square Types of sickle cell disease
Types of sickle cell disease Aplastic crisis
Aplastic crisis Sickle cell anemia
Sickle cell anemia Blood
Blood Software architecture of atm machine
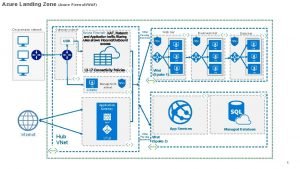
Software architecture of atm machine Azure zone region
Azure zone region Web security considerations
Web security considerations Final design icon
Final design icon Contrasting acquisition
Contrasting acquisition Pre-analytical considerations in phlebotomy
Pre-analytical considerations in phlebotomy Ethical consideration
Ethical consideration Slim cd inc
Slim cd inc Non rebreather mask nursing considerations
Non rebreather mask nursing considerations