Analysis Of Food 1 Introduction Food analysis is












































- Slides: 55

Analysis Of Food

1 -Introduction Food analysis is the discipline dealing with the � development, application and study of analytical � procedures for characterizing the properties of � foods and their constituents Characteristics of food, include their composition structure and physicochemical properties � Food components e. g. lipids, proteins, water, carbohydrates and minerals � � �

2 - Food Safety � -one of the most important reasons for analyzing food to ensure that they are safe -Food considered to be unsafe because it may contains: 1 - harmful micro organisms (Salmonella). 2 - toxic chemicals (pesticides). 3 - extraneous matter(glass, wood, metal, insects and matter) So analytical techniques must be of high sensitivity to detect low levels of harmful material

Properties Analyzed 1 - Composition: a- most foods are compositionally complex material Made up of a variety of different chemical constituents b- Composition depends upon the property that is of interest to the analyst e. g. : specific atoms : C, H, N, O, S, Na, …etc specific molecules : water, sugar, …. Specific substances : milk, flour, butter, …. Types of molecules : fats, proteins, carbohydrates, minerals

2 -Structure a- Molecular structure(~1 -100 nm) type of molecules present b- Microscopic structure (~10 nm-100 μ m) food can be observed by microscopy (not by unaided eye) eg emulsion droplets, fat crystals, protein aggregates, . . . c- Microscopic structure (~> 100 μm) food can be observed by the unaided eye) eg sugar granules , raisons, chocolate chips food….

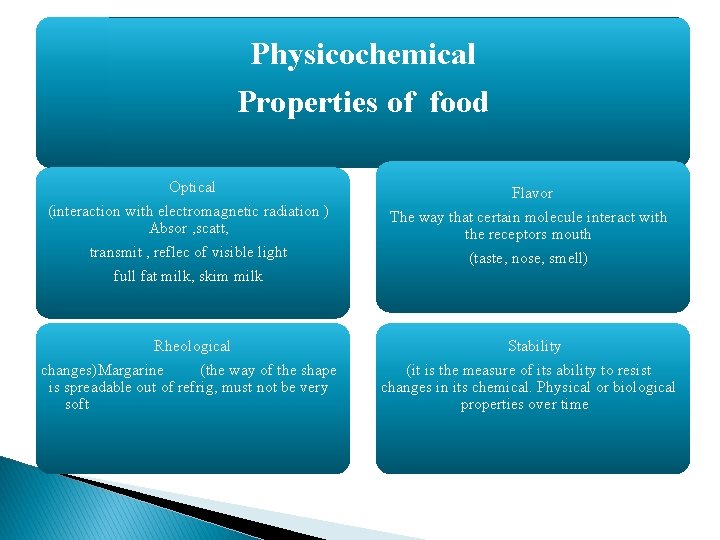
Physicochemical Properties of food Optical (interaction with electromagnetic radiation ) Absor , scatt, transmit , reflec of visible light full fat milk, skim milk Flavor The way that certain molecule interact with the receptors mouth (taste, nose, smell) Rheological changes)Margarine (the way of the shape is spreadable out of refrig, must not be very soft Stability (it is the measure of its ability to resist changes in its chemical. Physical or biological properties over time

Thus food must be carefully designed so that they have the required physicochemical properties over range of environmental conditions and Analytical techniques are needed to test properties

Food components: can be distinguished from each other by Molecular Characteristics: size , shape, polarity, interaction with radiation, …. (a physical properties: melting points, boiling points, density, …. Chemical reaction: Specific chemical (c reaction between certain component and an added reagent (b

3 - Selecting an Appropriate Technique Some of the criteria that are important in selecting a technique are: precision Accuracy Cost Simplicity of operation Reproducibility Sensitivity Speed destructive /Nondestructive safety

and Specify : a measure of ability to detect � quantify a specific components within a food material even in the presence of other similar components eg fructose in presence of sucrose or glucose

4 - Sample Selection and Sampling A food analyst has to select an appropriate fraction of the whole material which is one of the most important stages of food analysis , and can lead to large errors when not carried out correctly

5 -Prepartion of Laboratory Samples 5. 1 - Making Samples Homogeneous 1 - Mechanical devices e. g. , grinder, mixers , slicers , blenders 2 -Enzymatic method e. g. , professes, calluses, lipases 3 - chemical methods e. g. , strong acid , strong bases.

5. 2 -Reducing Sample Size and Preventing Changes in sample Enzymatic Inactivation Lipid Protection Microbial Growth and Contamination Physical changes

Analysis of Proteins are polymers of amino acid � Twenty different type f amino acid occur � naturally in proteins. Proteins differ from each other according to number and sequence of amino acid that makes up polypeptide backbone Thus proteins have different molecular � structure �

Proteins are important constituents of food, they are major source of energy. Proteins are major structural component of many natural food e. g. meat or fish product Protein are used as galling agent, emulsifiers, foaming agent. Many food proteins are enzymes which are capable of enhancing the rate off certain biochemical reaction Food analysis are interested in knowing the total concentration , type, molecular structure, and functional properties of proteins in food - -

Proteins Amino acids are the basic structural units of proteins. An amino acid is a compound that amino group(-NH 2) and contains at least one carboxyl group (-COOH)


Elements in Proteins



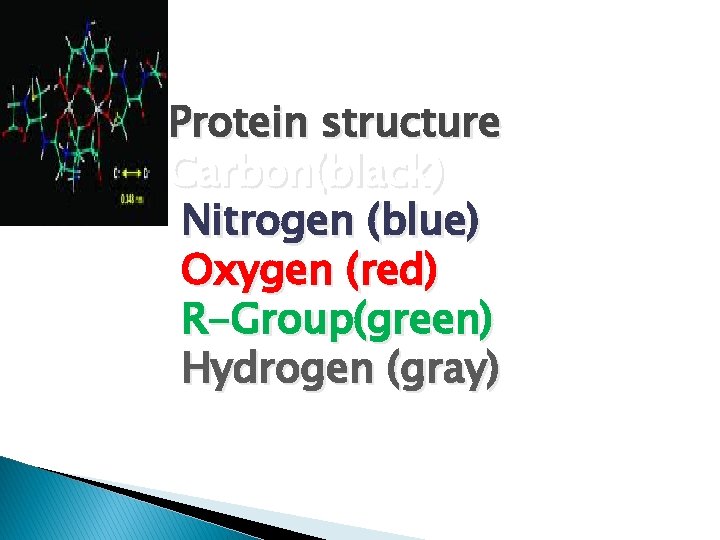
Protein structure Carbon(black) Nitrogen (blue) Oxygen (red) R-Group(green) Hydrogen (gray)

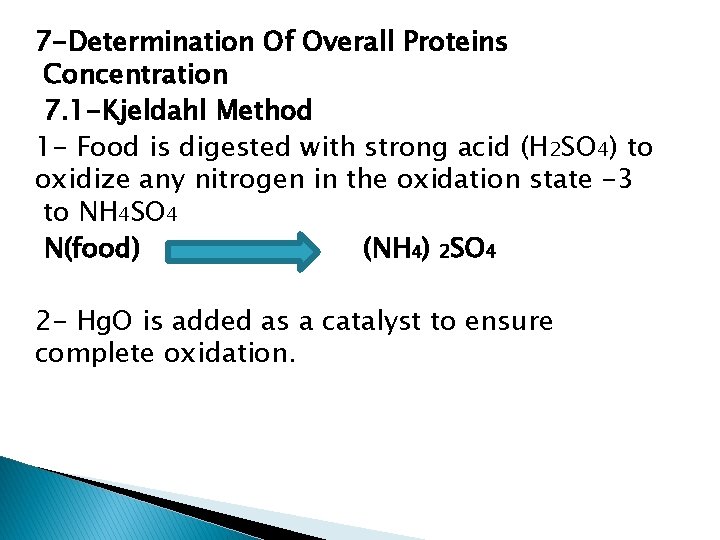
7 -Determination Of Overall Proteins Concentration 7. 1 -Kjeldahl Method 1 - Food is digested with strong acid (H 2 SO 4) to oxidize any nitrogen in the oxidation state -3 to NH 4 SO 4 N(food) (NH 4) 2 SO 4 2 - Hg. O is added as a catalyst to ensure complete oxidation.

3 - Nitrogen in oxidation states other than -3 (nitro, azo- are oxidation to N 2 resulting an error , salicylic acid is added as a reducing nitrogen to -3 state. 4 - The ammonium sulphate is then converted into ammonia gas by heating with sodium hydroxide (NH 4)2 SO 4+2 Na. OH Na 2 SO 4+2 H 2 O+2 NH 3

5 -NH 3 is collected in a flask containing a known amount of standard HCL. 2 NH 3+2 HCL 2(NH 4)CL 6 -The excess HCL is then titrated with standard Na. OH 1 Mole HCL=1 Mle N=14 g. N 7 -A blank sample is usually run at the same time as the material being analyzed to into account ay present into the residual nitrogen take may be reagent used to carry out the analysis. 8 - the continent of protein is equivalent to the amount of nitrogen found

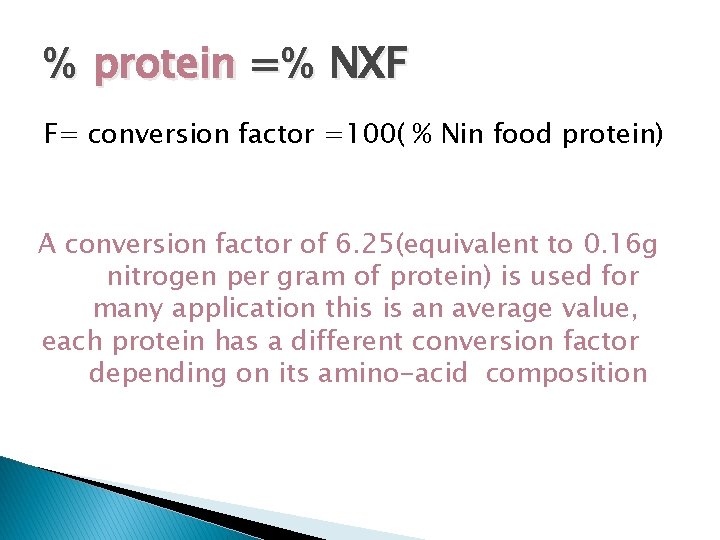
% protein =% NХF F= conversion factor =100( % Nin food protein) A conversion factor of 6. 25(equivalent to 0. 16 g nitrogen per gram of protein) is used for many application this is an average value, each protein has a different conversion factor depending on its amino-acid composition

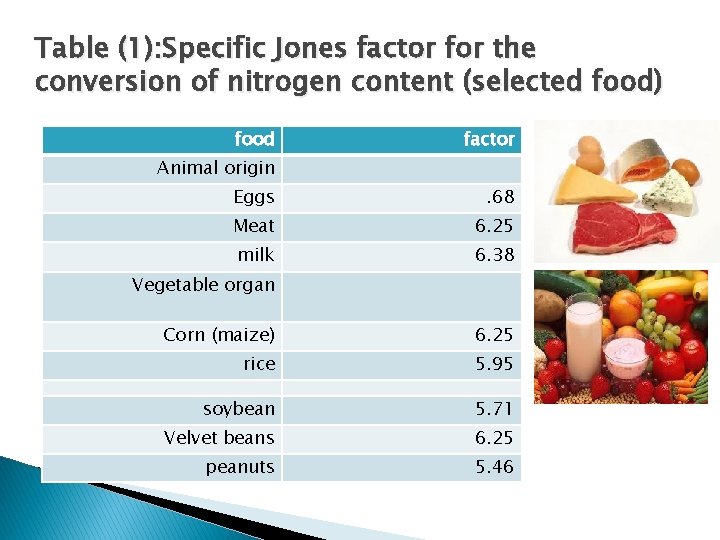
Table (1): Specific Jones factor for the conversion of nitrogen content (selected food) food factor Animal origin Eggs . 68 Meat 6. 25 milk 6. 38 Vegetable organ Corn (maize) 6. 25 rice 5. 95 soybean 5. 71 Velvet beans 6. 25 peanuts 5. 46

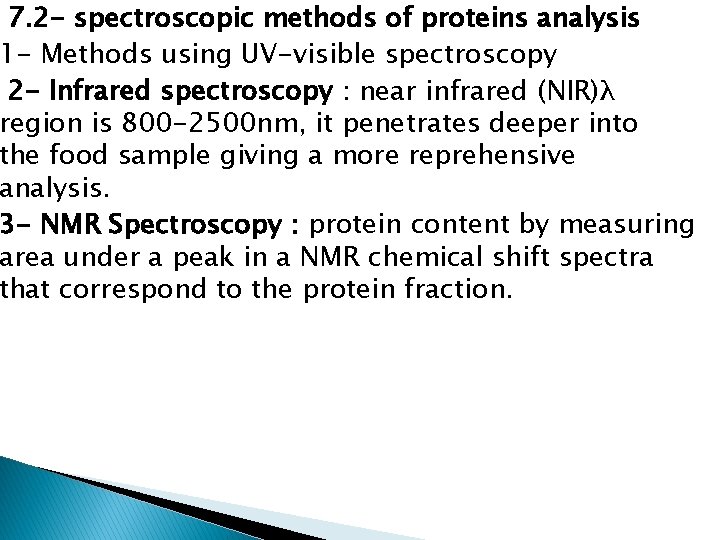
7. 2 - spectroscopic methods of proteins analysis 1 - Methods using UV-visible spectroscopy 2 - Infrared spectroscopy : near infrared (NIR)λ region is 800 -2500 nm, it penetrates deeper into the food sample giving a more reprehensive analysis. 3 - NMR Spectroscopy : protein content by measuring area under a peak in a NMR chemical shift spectra that correspond to the protein fraction.

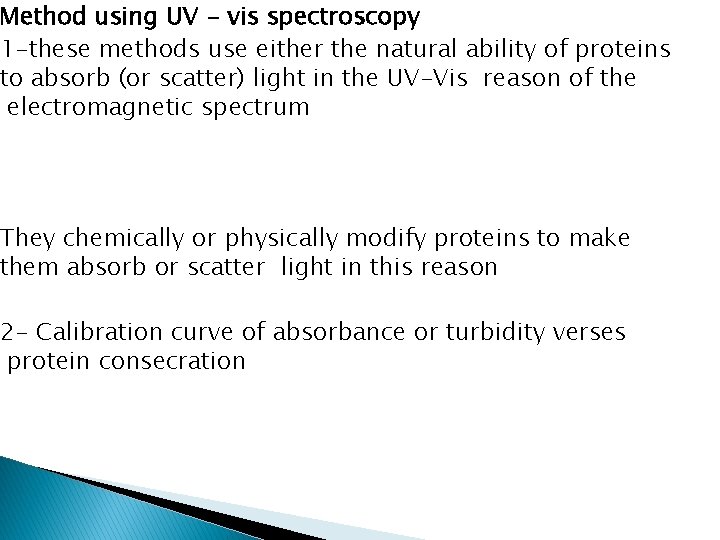
Method using UV - vis spectroscopy 1 -these methods use either the natural ability of proteins to absorb (or scatter) light in the UV-Vis reason of the electromagnetic spectrum They chemically or physically modify proteins to make them absorb or scatter light in this reason 2 - Calibration curve of absorbance or turbidity verses protein consecration

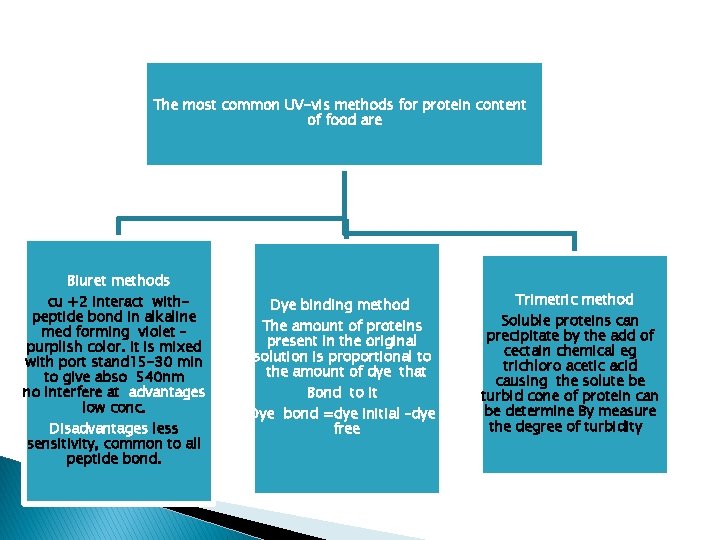
The most common UV-vis methods for protein content of food are Biuret methods cu +2 interact withpeptide bond in alkaline med forming violet – purplish color. It is mixed with port stand 15 -30 min to give abso 540 nm no interfere at advantages low conc. Disadvantages less sensitivity, common to all peptide bond. Dye binding method The amount of proteins present in the original solution is proportional to the amount of dye that Bond to it Dye bond =dye initial –dye free Trimetric method Soluble proteins can precipitate by the add of cectain chemical eg trichloro acetic acid causing the solute be turbid cone of protein can be determine By measure the degree of turbidity

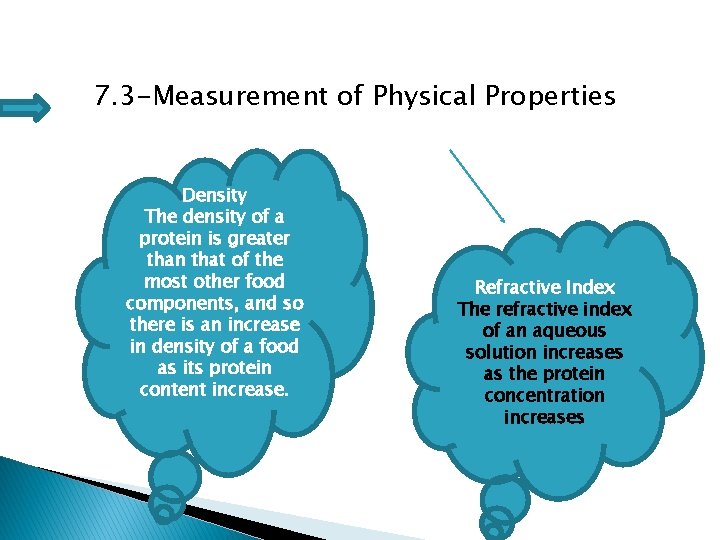
7. 3 -Measurement of Physical Properties Density The density of a protein is greater than that of the most other food components, and so there is an increase in density of a food as its protein content increase. Refractive Index The refractive index of an aqueous solution increases as the protein concentration increases

8 - protein separation and characterization 1 -Separation due to Different Adsorption characterization Ion exchange chromatography. 2 -Separation due to size difference. Size exclusion chromatography 3 - Separation by electrophoresis

9 - Metal in food Tin Zinc Arsenic Copper These metals can be determine gravimetrically or by atomic absorption methods.

9 -1 MERCURY IN FOOD PRODUCT Mercury in mushroom Mercury in fishes

1 - fish is an important part of a healthy diet 2 - benefits of fish: high in protein, law in saturated fat and high in unsaturated fat 3 - most people are exposed to mercury via food 4 - fish takes up mercury from steams and oceans as they feed, this mercury is the most toxic form , methyl mercury. It binds to there tissue protein (muscles)

5 - there are three forms of mercury : organic , inorganic and metallic. 6 -organic form I dangerous (methyl mercury). 7 - inorganic mercury is converted to organic mercury by anaerobic bacteria at the bottom of lakes streams 8 - small aquatic animals consumes the organic mercury, and in turn eaten by larger life form. 9 -as the elements move up to the food chain , forms microbes to the fish then to large animal as swordfish, mercury becomes more concentrated.

10 - oysters may concentrate mercury by a factor of 100, 000. 11 -mercury levels differs from one species of fish to the next , according to fish, size, location, habitat and age. sh contains higher levels of mercury includes , shark , swordfish , barramundi, … 13 - fish contains lower levels of and canned tuna.


9. 1. 1 Analysis of mercury in fish 1. Atomic Absorption Methods 2. Dithizone Colorimetric Procedure 3. Neutron Activation Analysis

Dithizone Colorimetric Procedure 1 - It is the most commonly used method. 2 - wet oxidation process. 3 - Hydroxylamine hydrochloride is added to reduce the remaining oxidizing material from wet oxidization. 4 -Mercury is extracted from solution using Dithizone in chloroform. 5 - Copper can interact with thiazone causing interference

6 - Sodium thiosulfate is added to the CHC 13 layer, a water soluble mercury thiosulfate is formed while copper Dithizone remains in CHC 13 7 - The aquophase containing mercury-thiosulfate is again oxidizes by H 2 SO 4 or HNO 3 and mercury is extracted with in CHC 14 8 - Mercury – dithionate is determined spectrophotometrically at 490 nm

“Advantages”: =Low cost, simplicity, senility. “Disadvantages”: Interference of copper.

fffffffff 10 -carbohydrates in food Total carbohydrate content of food is calculated by difference , rather than analyzed directly. Thus the constituents in food (proteins, fats water, ash, alcohol) are determined individually , summed and subtracted from total weight of the food. Total cryohydrate = 100(weight in grams(protein, fat, water, ash, alcohol) in 100 grams of food

11 -Separation and identification of sugars A- Paper chromatography b- high performance liquid chromatography Example separation of fructose , orbital , scarce and lactose 1 - fructose 2 - orbital 3 - scarce 4 - lactose

12 - fats in food Fats are hydrolyzed using an alkali such as(Na. OH), the alcohol is liberated and also salts of fatty acids (soaps)

1 - Fats become rancid as a result of peroxide formation at the double bonds by atmospheric oxygen and hydrolysis by micro organisms with the liberation of free acids. 2 - The amount of free acids presents gives an indication of the age and quality of fats 3 - Acids value : is the number of milligrams of KOH requires to neutralize the free acid present in 1 gm fat.

12. 1 Separation and Charactration of Free Fatty Acids From Milk 1 -The milk sample is prepared by mixing 10 ml of milk 10 ml, of 28%ammonium hydroxide, 25 ml petroleum ether and 25 ml diethyl ether.

2 -when the mixture is well shaken it allows to stands 20 min, the residue is treated with 3 ml 0. 5 N Na. OH in methanol, heated on a stream bath 15 min. 3 - 5 ml water is added then 2 N HCL till p. H is about 2 is reached. 4 - Separation was carried out using Gas Chromatographic G. C) method.

5 - Glass column , 3 m long , 2 mm diameter, packed with silicon polymer. 6 - The column is then programmed from 130 -200 ˚C Nitrogen is used as carrier gas. 7 - This method is effective, rapid, accurate uantitative results were easily obtained

The separation of the fatty acid is milk 1/n-Valeric acid 2/n Caproic acid 3/n-Caprylic acid 4/n- Capric acid 5/n-Lauric acid 6/n-Myristic acid 7/n-Palmitic acid 8/n-Stearic acid 9/n-c 16 -1 ene 10/n- Oleic acid 11/n-Linoleic acid 12/nlinolenicacid

Flavor and Fragrances

Flavor is defined as a substance that gives another substance flavor, altering the characteristics of the solute, casing it to become sweet, sour, etc The flavor of the food, can be natural or artificial flavoring Due to the high or unavailability of natural flavor extracts most commercial flavoring are nature-identical which means that they are the chemical equivalent of natural flavors but chemically synthesized rather than being xtracted from the source material

Certain organic acids can be used to enhance sour tastes, Each acids impart a slightly different sour avor of a food Acetic acids: gives vinegar its sour taste and Citric acids: found in citrus fruits and gives then them their sour taste. Lactic acids: found in various milk product and give them rich tartness Malic acids: found in apples that gives them their sour/taste. Tartaric acids: found in grapes and wines and gives hem a tart taste

A- The accurate and rapid identification and quantitation of flavor and fragrance compounds are often limited by the methods use for extracting, collecting, and concentrating analyte species prior to/gas chromatographic analysis avors.

Liquid solvent extraction, steam distillation, methods –B are frequely used for the extration and collection of freagrance and flavor compounds but can require several hours to perform, may yield low extraction efficiencies and may result in loss or degradation of nalyte species.

GOOD LUCK DONE BY:
 Unit 2 food food food
Unit 2 food food food Sequence of food chain
Sequence of food chain Essay structure
Essay structure Topicfast
Topicfast Introduction for fast food
Introduction for fast food The flow of food: an introduction
The flow of food: an introduction Module 1 introduction to food safety
Module 1 introduction to food safety Introduction of food hygiene
Introduction of food hygiene Food production introduction
Food production introduction Introduction to food safety answers
Introduction to food safety answers Introduction to food and beverage service department
Introduction to food and beverage service department Introduction to food and beverage service department
Introduction to food and beverage service department Introduction to food and beverage service
Introduction to food and beverage service Introduction about food presentation
Introduction about food presentation Introduction to food and beverage service department
Introduction to food and beverage service department Food web 7th grade science
Food web 7th grade science Indirect contact freezing example
Indirect contact freezing example Food scientist measure food energy in
Food scientist measure food energy in Importance of food
Importance of food Tcs food
Tcs food A little food or a few food
A little food or a few food Primary consumer
Primary consumer Food handlers can contaminate food when they answer
Food handlers can contaminate food when they answer Food product design summary
Food product design summary The many overlapping food chains in an ecosystem
The many overlapping food chains in an ecosystem Food web producers
Food web producers How does the food chain go
How does the food chain go Fast food can be defined as any food that contributes
Fast food can be defined as any food that contributes Http //www.harcourtschool.com/activity/food/food menu.html
Http //www.harcourtschool.com/activity/food/food menu.html 4 food chains
4 food chains Food chain for kids
Food chain for kids Bnf explore food
Bnf explore food Junk food vs healthy food project
Junk food vs healthy food project Food chains, food webs and ecological pyramids
Food chains, food webs and ecological pyramids Food chain and food web examples
Food chain and food web examples Food webs and energy pyramids answer key
Food webs and energy pyramids answer key Chaparral food chains
Chaparral food chains How many food chains are there in the food web
How many food chains are there in the food web Role play on healthy food and junk food
Role play on healthy food and junk food Of junk food
Of junk food Junk vs healthy food
Junk vs healthy food Food web of the desert
Food web of the desert Piramid makanan
Piramid makanan Junk food
Junk food Control measures for physical hazards
Control measures for physical hazards Food resources world food problems
Food resources world food problems Food a fact of life
Food a fact of life Food handlers can contaminate food when they
Food handlers can contaminate food when they To prevent food allergens from being transferred to food
To prevent food allergens from being transferred to food Sad system analysis and design
Sad system analysis and design Introduction to nonlinear analysis
Introduction to nonlinear analysis Hunger games analysis essay
Hunger games analysis essay Waterski across the surface of a poem
Waterski across the surface of a poem Algorithm analysis examples
Algorithm analysis examples Nucleation in gravimetric analysis
Nucleation in gravimetric analysis Affiliation audience analysis
Affiliation audience analysis