AIR POLLUTION Primary and Secondary Pollutants p H























- Slides: 52

AIR POLLUTION Primary and Secondary Pollutants; p. H

Types of Pollutants • Primary pollutants – those released directly into the lower atmosphere, i. e. CO • Secondary pollutants – those that are formed by the combination of primary pollutants in the atmosphere. For example, acid rain is produced from the combination of sulfur oxides (SO 2 and SO 3) and water vapor. – Other examples: HNO 3, H 2 O 2, H 2 SO 4. PANs, NO 3 -, SO 4 -

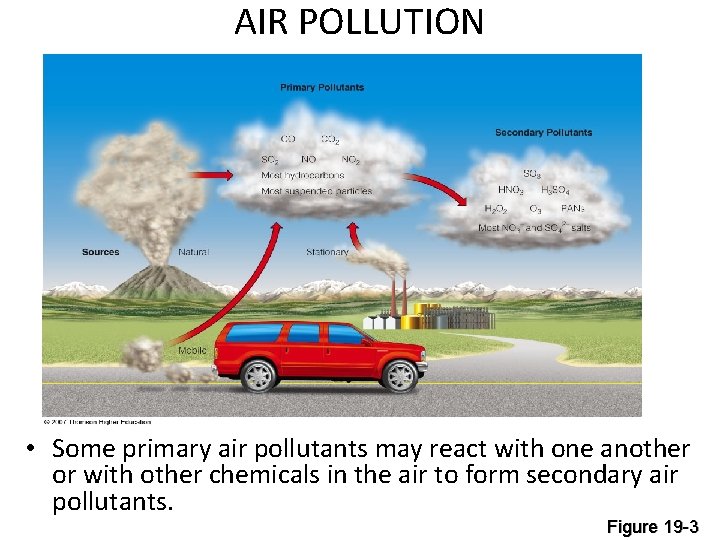
AIR POLLUTION • Some primary air pollutants may react with one another or with other chemicals in the air to form secondary air pollutants. Figure 19 -3

How Do They Form and What Are the Sources • How are secondary pollutants formed? • Describe the sources of primary pollutants? Answers: 1. Primary air pollutants react with one another or with other chemicals in the atmosphere and form secondary pollutants. 2. Mobile exhaust, natural pollutants (volcanic ash, etc. ), and stationary pollutants from refineries and processing plants.

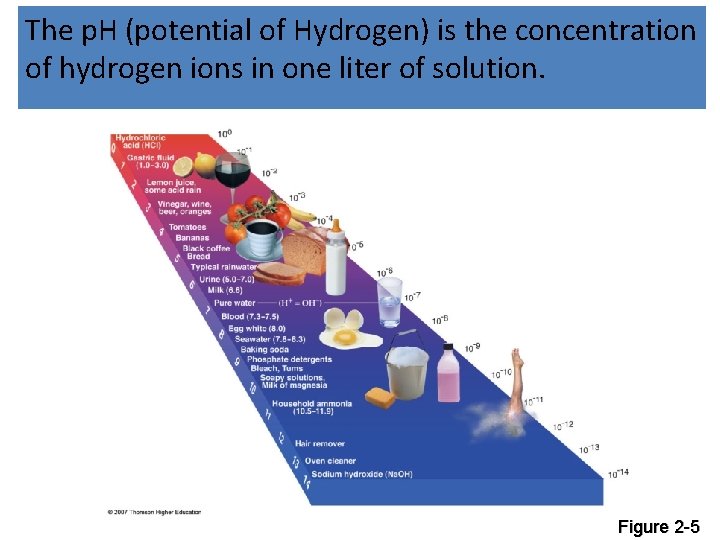
The p. H (potential of Hydrogen) is the concentration of hydrogen ions in one liter of solution. Figure 2 -5

Formation of Acid Deposition • The sequence of events which result in acid deposition formation are 1. Combustion releasing SO 2 and NOx 2. Secondary pollutants are formed 3. Dissociation of pollutants 4. Deposition of ions on vegetation or soil

Other Acid Rain Factoids • Normal rainfall has a p. H of 5. 7 • Typical rainfall in eastern US has a p. H of 4. 6 • In general, acid rain has harmful effects for terrestrial ecosystems when it falls below a p. H of 5. 6 • Linked to NOx and SO 2 • Fish kills; damages or kills aquatic life • Stunts plant growth and makes them susceptible to disease • Damages statues, buildings and car finishes

General Terms • AQI (Air Quality Index) – measurement of air quality; usually in parts per million (ppm). • National Ambient Air Quality Standards – specify the maximum allowable level, averaged over a specific time period, for a certain pollutant. • Point source pollution describes a specific location from which pollution is released; i. e. , factory or forest fire. • Non-point source pollution – pollution that does not have a specific point of release; i. e. , methane from cows, automobiles.

Behavior in the Atmosphere • Humans have added pollutants to the air throughout history (early man’s fire, Roman’s smelting lead); [anthropogenic]. However, large-scale production of pollutants began with the Industrial Revolution. • Today, idling cars add most air pollution………”Welcome to Mc. Donald’s; may I take your order? ” • Once air pollutants have entered the atmosphere, they may be 1. Removed with precipitation 2. Transformed through chemical reactions 3. Transported on air currents and wind

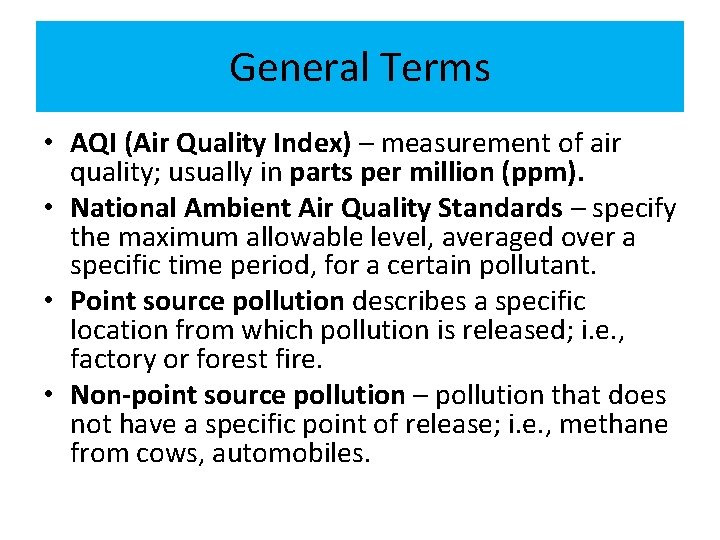
Temperature Inversion • Temperature inversions trap pollutants close to the Earth’s surface and exacerbate pollution problems. • Often occur at night • Result from 1. 2. 3. Warm air on top of cooler, stagnant air Movement of a cold front From infiltration of ocean air by a cooler onshore breeze

Thermal Incline • Common in 1. Coastal areas – LA 2. Areas located in basins – LA & Mexico City 3. Valleys surrounded by mountains – Smokey Mtns.

Affect on Humans • London (1952) – 4, 000 deaths – London (1880) – 700 deaths – London (1892) – 1, 000 deaths • “killer fog” resulted from burning large quantities of coal • Donora, PA (1948) – 20 deaths; 7, 000 sickened – Fluoride emissions from US Steel Co. • New York City (1963) – 300 deaths; thousands of illnesses

Smog • Industrial (gray) smog – Results from incomplete combustion of fossil fuels such as coal, oil and natural gas – Releases CO, CO 2, soot, sulfur and Hg – Reacts with oxygen and sunlight • Photochemical (brown) smog – Formed from reactions involving nitrogen oxides – These reactions also produce acid rain and tropospheric ozone


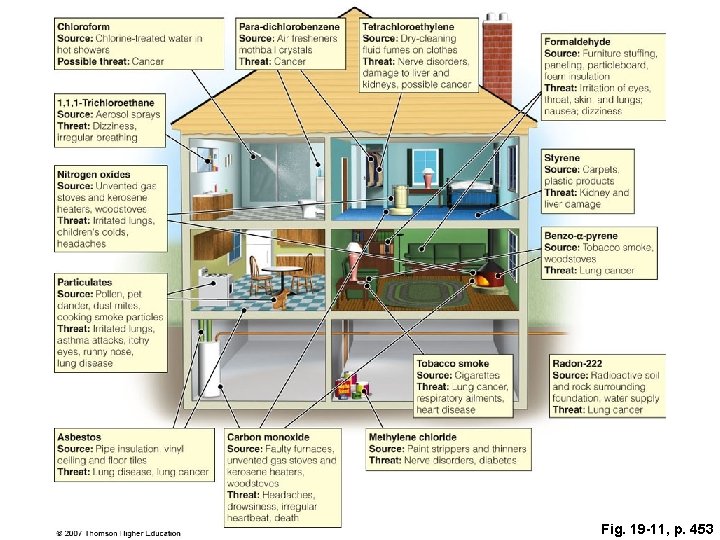
Indoor Air Pollution

Fig. 19 -11, p. 453

Factoids • Asbestos, radon-222, formaldehyde and cigarette smoke – 4 most dangerous • Dust mites, cockroach droppings, mold & mildew – contributors to asthma

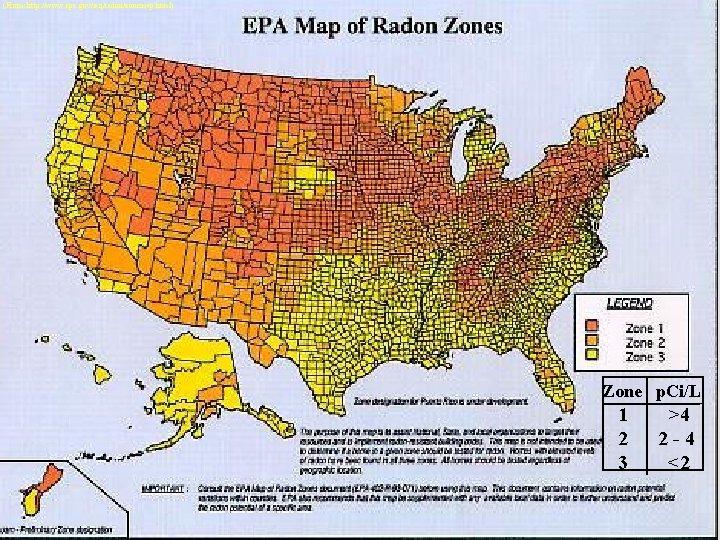
Radon • 55% of our exposure to radiation comes from radon • Strong carcinogen • colorless, tasteless, odorless gas • formed from the decay of uranium-238 • found in nearly all soils, igneous rock • levels vary geographically • Remediation includes sealing or venting areas

(From: http: //www. epa. gov/iaq/radon/zonemap. html) Zone p. Ci/L 1 2 3 >4 2 -4 <2

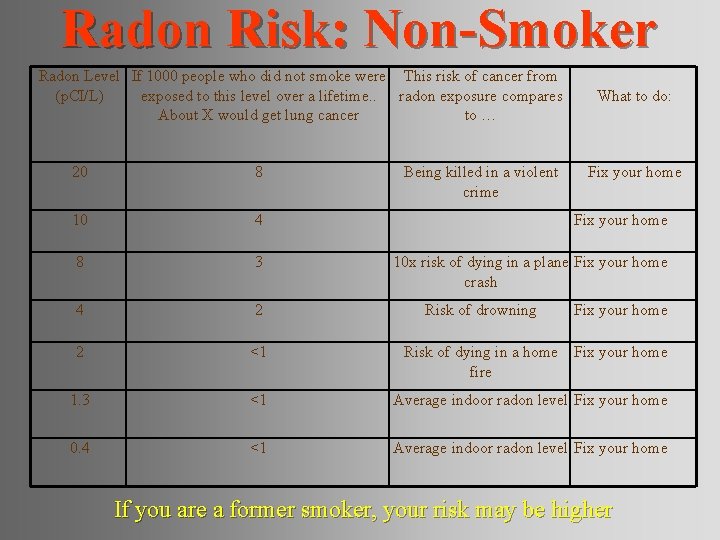
Radon Risk: Non-Smoker Radon Level If 1000 people who did not smoke were This risk of cancer from (p. CI/L) exposed to this level over a lifetime. . radon exposure compares About X would get lung cancer to … Being killed in a violent crime What to do: 20 8 10 4 Fix your home 8 3 10 x risk of dying in a plane Fix your home crash 4 2 2 <1 Risk of dying in a home Fix your home fire 1. 3 <1 Average indoor radon level Fix your home 0. 4 <1 Average indoor radon level Fix your home Risk of drowning Fix your home If you are a former smoker, your risk may be higher

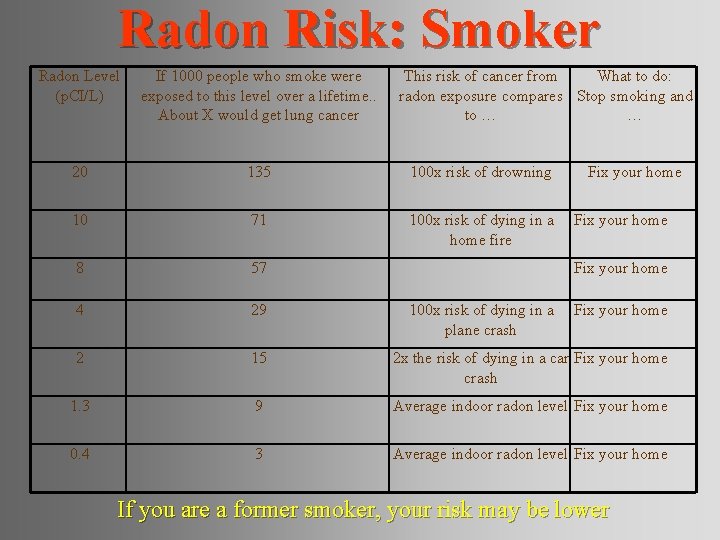
Radon Risk: Smoker Radon Level (p. CI/L) If 1000 people who smoke were exposed to this level over a lifetime. . About X would get lung cancer This risk of cancer from What to do: radon exposure compares Stop smoking and to … … 20 135 100 x risk of drowning 10 71 100 x risk of dying in a home fire 8 57 4 29 2 15 2 x the risk of dying in a car Fix your home crash 1. 3 9 Average indoor radon level Fix your home 0. 4 3 Average indoor radon level Fix your home 100 x risk of dying in a plane crash Fix your home If you are a former smoker, your risk may be lower

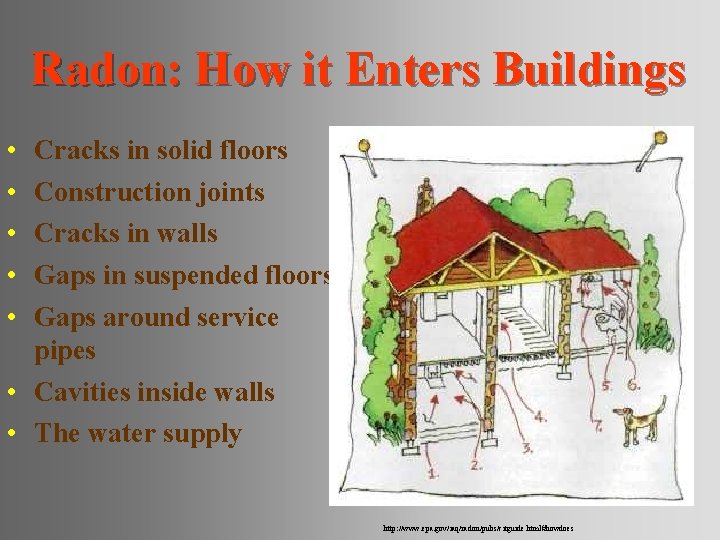
Radon: How it Enters Buildings • • • Cracks in solid floors Construction joints Cracks in walls Gaps in suspended floors Gaps around service pipes • Cavities inside walls • The water supply http: //www. epa. gov/iaq/radon/pubs/citguide. html#howdoes

Radon: Reducing the Risks • Sealing cracks in floors and walls • Simple systems using pipes and fans for ventilation

EPA Studies Concluded 1. Levels of 22 common pollutants are generally higher inside US homes and commercial buildings than outdoors 2. Indoor pollutants may be linked to Sick Building Syndrome 3. Pollution levels inside cars in traffic-logged areas are higher than outside 4. Health risks from exposure to chemical pollutants are higher for people in developed countries because they spend more time indoors or inside vehicles

Sick Building Syndrome (SBS) vs Building Related Illness (BRI)

Sick Building Syndrome • A building is considered “sick” when at least 20% of its occupants suffer persistent symptoms that disappear when they exit the building • Causes(s) not known or recognizable • At least 17% of the 4 million commercial buildings in the US are considered “sick”

Complaints/Symptoms • • • Dry Skin Nasal Congestion Difficulty Breathing Nose Bleeds Nausea


Legislation • Air Pollution Control Act (1955) – Precursor to CAA – Awareness; national problem (Donora, PA; LA, London) • Clean Air Act (1963) – begins regulation of air pollution Established Air Quality Control regions Set air quality standards for public health Developed motor vehicles emissions standards Set point source (stationary) emissions standards for all new facilities – Gave a time table to power plants to cut emissions – Established a cap-and-trade program for SO 2 in 1990 – –

Amendments • CAAA (Clean Air Act Amendments) – made law stricter • 1965 • 1970 – unrealistic; caused economic hardship for companies, so……. . • 1990 – allowed companies to use “Best Available Control Technologies” – best tech they could afford; emphasis on alternative fuels • Does NOT regulate CO 2 emissions (GHG)

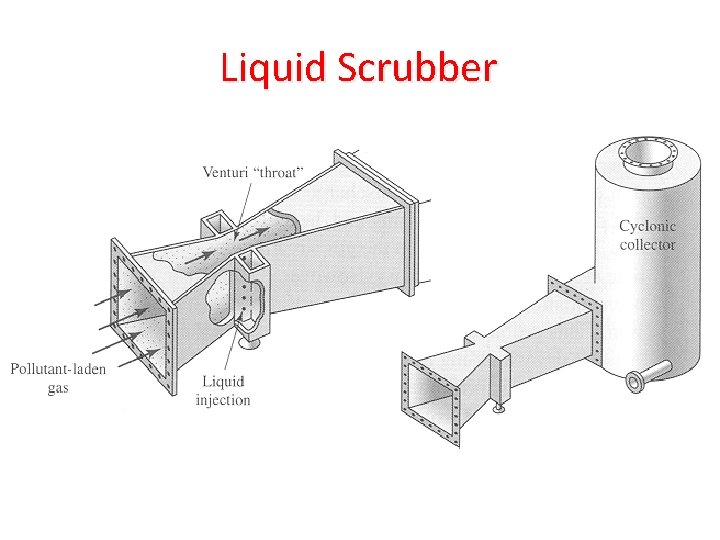
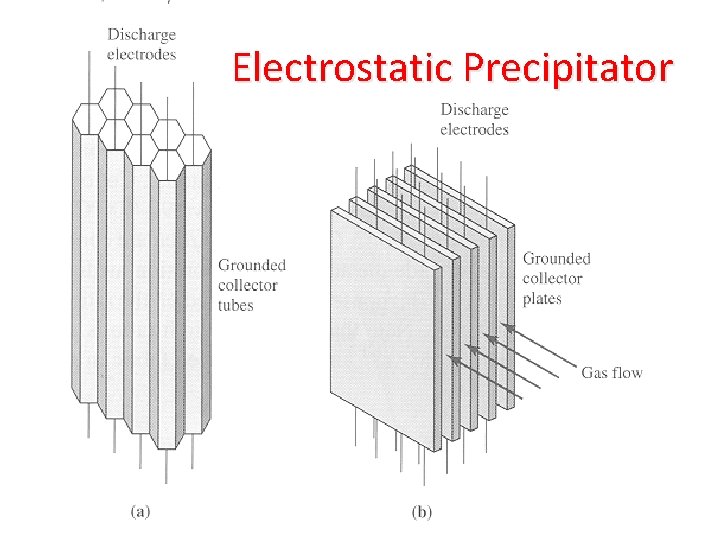
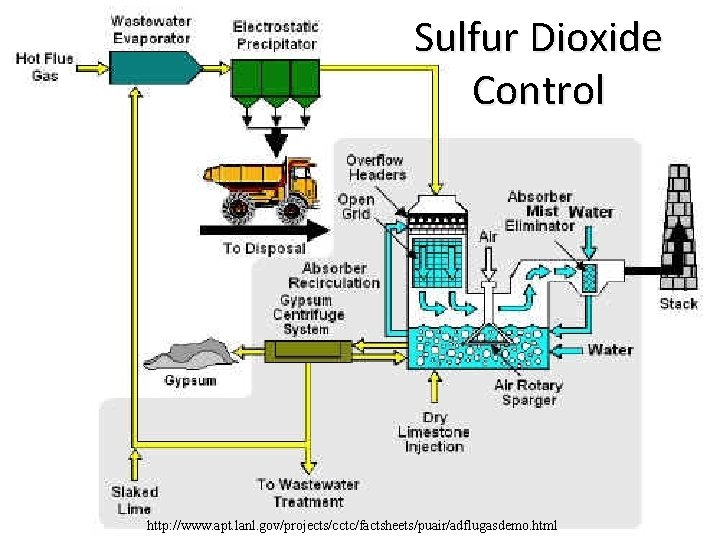
Strategies to Reduce Air Pollutants 1. Removing sulfur from coal 2. Burning low-sulfur coal 3. Convert coal to liquid or gaseous fuel (cleaner burning) 4. Shifting to less polluting fuels 5. Use emission-control devices – Wet-scrubbers – decrease SO 2 from coal-burning power plants – Electrostatic precipitators – remove particulates from smokestacks – Baghouse filters – Cyclone separators

Liquid Scrubber

Electrostatic Precipitator

Sulfur Dioxide Control http: //www. apt. lanl. gov/projects/cctc/factsheets/puair/adflugasdemo. html

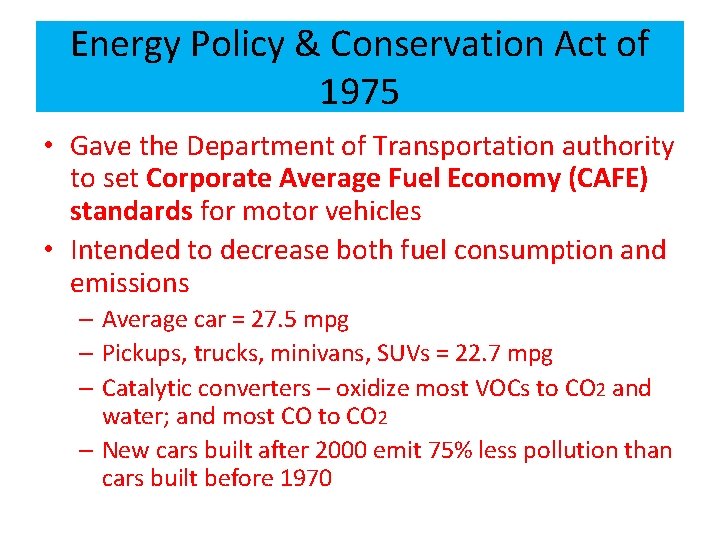
Energy Policy & Conservation Act of 1975 • Gave the Department of Transportation authority to set Corporate Average Fuel Economy (CAFE) standards for motor vehicles • Intended to decrease both fuel consumption and emissions – Average car = 27. 5 mpg – Pickups, trucks, minivans, SUVs = 22. 7 mpg – Catalytic converters – oxidize most VOCs to CO 2 and water; and most CO to CO 2 – New cars built after 2000 emit 75% less pollution than cars built before 1970

Milestones in the Control of Automotive Emissions 1952 - Autos linked to air pollution 1963 - Original CAA, PCV valves 1968 - HC & CO exhaust controls 1970 - CAA amendments, EPA formed 1971 - Evaporative controls 1972 - First I/M Program 1973 - NOx exhaust controls 1975 - First catalytic converters 1981 - New cars meet statutory limits 1989 - Volatility limits on gasoline 1990 - New CAA Amendments


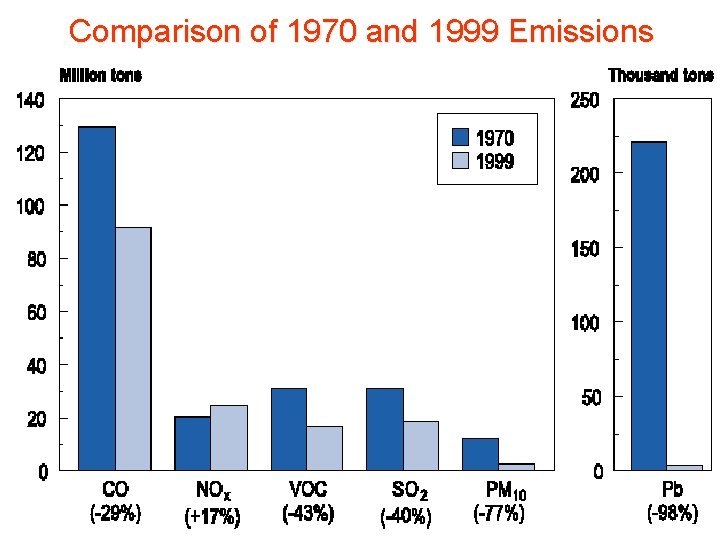
Comparison of 1970 and 1999 Emissions

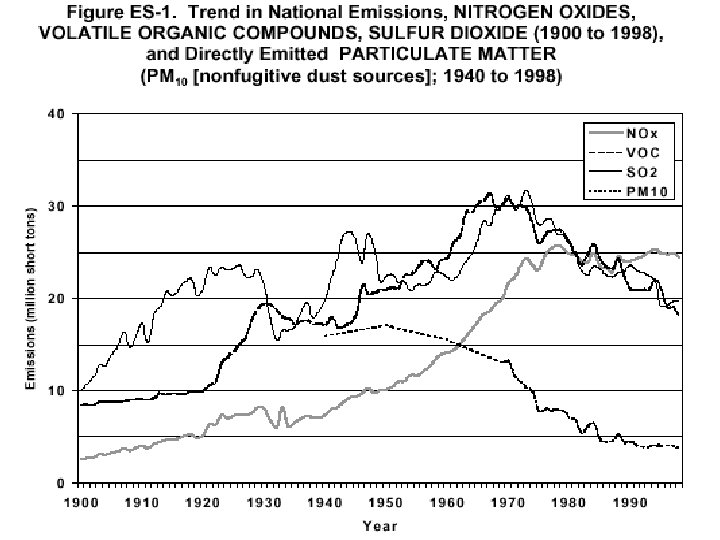
Reduction in Air Pollution • EPA report (2005) – reduction between 1970 & 2004 1. 2. 3. 4. Lead Suspended particulate matter CO SO 2

Question • What are some reasons why a multinational company would choose to build a manufacturing facility in India or China rather than in the US or Europe?

China • High levels of air pollution – burn lots of coal • No/few regulations • No scrubbers • Shut down industry just before and during 2008 Olympics

Other Primary Pollutants • CFCs (chlorofluorocarbons) – Invented in the 1930 s – Used as coolants, propellants, in fire extinguishers, and aerosols – Known to cause depletion of stratospheric ozone • Ozone depletion can lead to – – Eye cataracts Skin cancer Weakened immune systems Decrease in productivity of marine and terrestrial ecosystems • PCBs (polychlorinated biphenyls) – A group of 209 toxic, oily, synthetic chlorinated hydrocarbon compounds that can be biologically amplified in food chains/webs – Neurotoxin – Sources: pesticides, hydraulic fluids, transformers, wood treatments, paint, plastic, roofing materials, lubricants………………. .

Stratospheric Ozone • It is formed from the reaction of O with O 2 in the presence of ultraviolet light • Without interference, there is a steady state of ozone being created and destroyed • The ozone absorbs UV-B rays and decomposes into O 2 and O • It is a closed loop cycle

How Have We Depleted O 3 in the Stratosphere and What Can We Do? • Widespread use of certain chemicals has reduced ozone levels in the stratosphere, which allows for more harmful ultraviolet radiation( UV-A and UVB) to reach the earth’s surface. • To reverse ozone depletion, we must stop producing ozone-depleting chemicals and adhere to the international treaties that ban such chemicals.

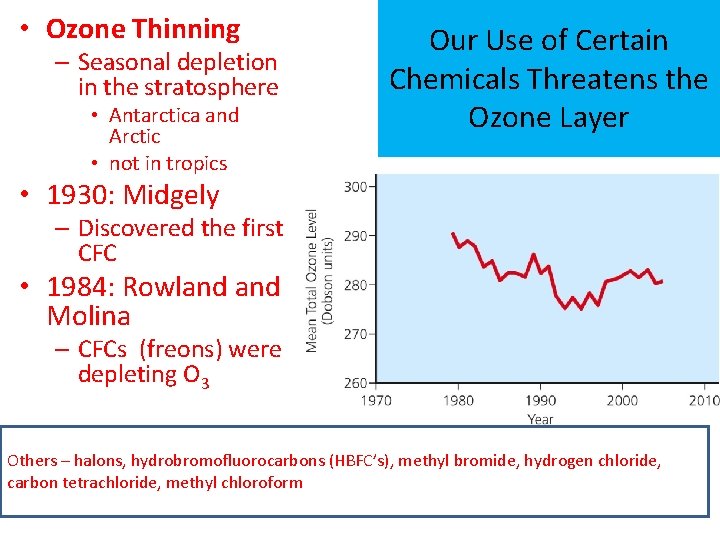
• Ozone Thinning – Seasonal depletion in the stratosphere • Antarctica and Arctic • not in tropics Our Use of Certain Chemicals Threatens the Ozone Layer • 1930: Midgely – Discovered the first CFC • 1984: Rowland Molina – CFCs (freons) were depleting O 3 Others – halons, hydrobromofluorocarbons (HBFC’s), methyl bromide, hydrogen chloride, carbon tetrachloride, methyl chloroform

Rowland Molina (1974)……. • Research – CFCs are persistent in the atmosphere – Rise into the stratosphere over 11 -20 years – Break down under high-energy UV radiation • Halogens produced accelerate the breakdown of O 3 to O 2 – Each CFC molecule can last 65 -385 years • 1988: Du. Pont stopped producing CFCs – stalled for 15 years(1974) • 1995: Nobel Prize in chemistry

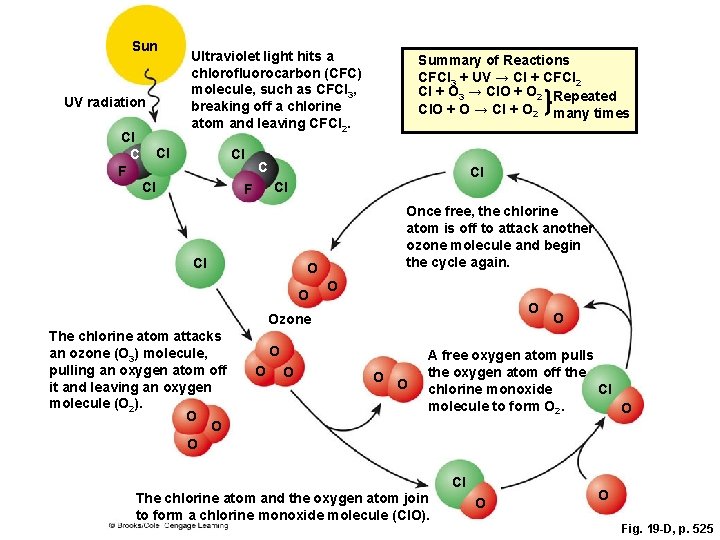
Sun UV radiation Cl C F Ultraviolet light hits a chlorofluorocarbon (CFC) molecule, such as CFCl 3, breaking off a chlorine atom and leaving CFCl 2. Cl Cl Cl Summary of Reactions CFCl 3 + UV → Cl + CFCl 2 Cl + O 3 → Cl. O + O 2 Repeated Cl. O + O → Cl + O 2 many times C Cl Cl F Cl Once free, the chlorine atom is off to attack another ozone molecule and begin the cycle again. O O Ozone The chlorine atom attacks an ozone (O 3) molecule, pulling an oxygen atom off it and leaving an oxygen molecule (O 2). O O O O O A free oxygen atom pulls the oxygen atom off the Cl chlorine monoxide molecule to form O 2. O Cl The chlorine atom and the oxygen atom join to form a chlorine monoxide molecule (Cl. O). O O Fig. 19 -D, p. 525

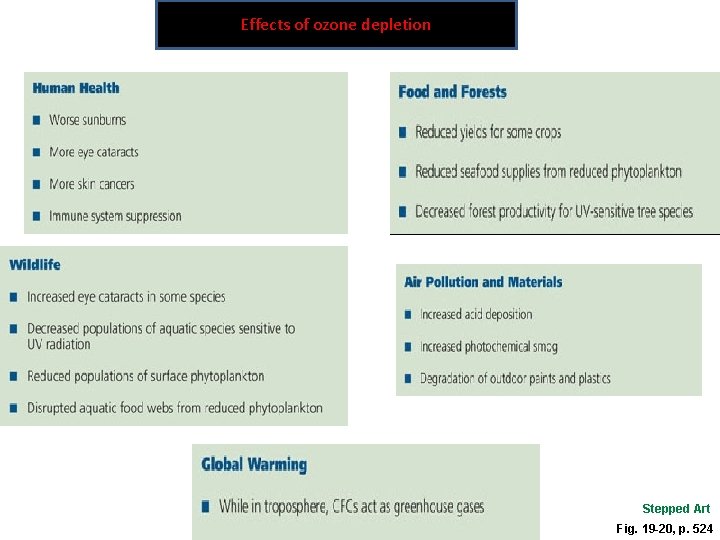
Why Should We Worry about Ozone Depletion • Damaging UV-A and UV-B radiation – Increase eye cataracts and skin cancer • Impair or destroy phytoplankton- Antarctic – base of food web • Loss of removal of carbon dioxide from the atmosphere – worsening global warming

Effects of ozone depletion Stepped Art Fig. 19 -20, p. 524

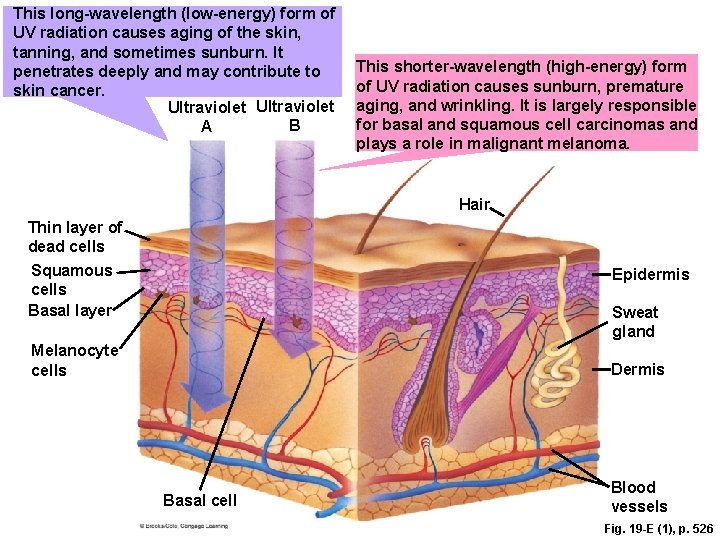
This long-wavelength (low-energy) form of UV radiation causes aging of the skin, tanning, and sometimes sunburn. It penetrates deeply and may contribute to skin cancer. Ultraviolet B A This shorter-wavelength (high-energy) form of UV radiation causes sunburn, premature aging, and wrinkling. It is largely responsible for basal and squamous cell carcinomas and plays a role in malignant melanoma. Hair Thin layer of dead cells Squamous cells Basal layer Epidermis Sweat gland Melanocyte cells Dermis Basal cell Blood vessels Fig. 19 -E (1), p. 526

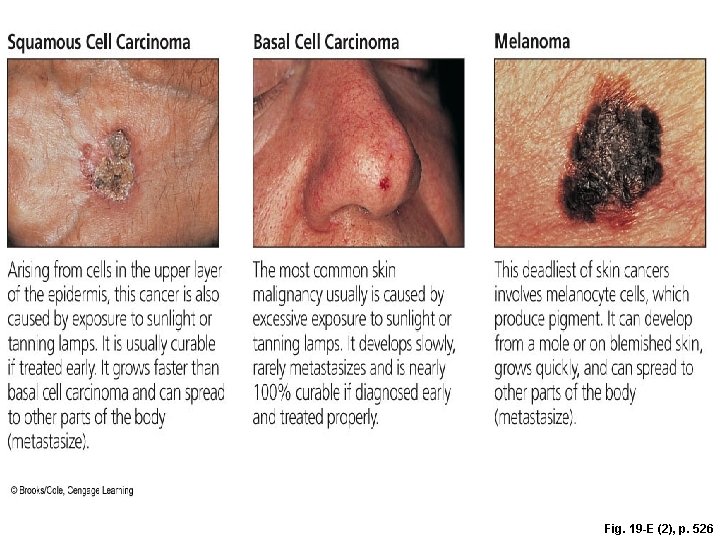
Fig. 19 -E (2), p. 526

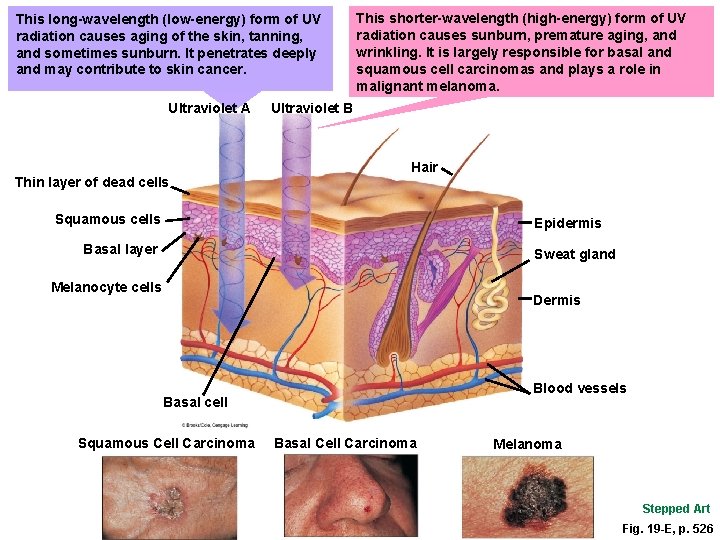
This long-wavelength (low-energy) form of UV radiation causes aging of the skin, tanning, and sometimes sunburn. It penetrates deeply and may contribute to skin cancer. Ultraviolet A Thin layer of dead cells This shorter-wavelength (high-energy) form of UV radiation causes sunburn, premature aging, and wrinkling. It is largely responsible for basal and squamous cell carcinomas and plays a role in malignant melanoma. Ultraviolet B Hair Squamous cells Epidermis Basal layer Sweat gland Melanocyte cells Dermis Blood vessels Basal cell Squamous Cell Carcinoma Basal Cell Carcinoma Melanoma Stepped Art Fig. 19 -E, p. 526
 Planting more trees is called
Planting more trees is called Primary pollutants and secondary pollutants
Primary pollutants and secondary pollutants Primary pollutant vs secondary pollutant
Primary pollutant vs secondary pollutant What are secondary pollutants
What are secondary pollutants Differentiate between primary and secondary pollutants
Differentiate between primary and secondary pollutants Primary and secondary pollutants difference
Primary and secondary pollutants difference Primary vs secondary pollution
Primary vs secondary pollution Primary vs secondary pollutants
Primary vs secondary pollutants What is secondary pollutant
What is secondary pollutant What are the secondary air pollutants
What are the secondary air pollutants Secondary pollutants examples
Secondary pollutants examples Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Major air pollutants
Major air pollutants Air pollutants
Air pollutants Indoor air pollution sources
Indoor air pollution sources Chapter 12 air section 1
Chapter 12 air section 1 Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Air higroskopis adalah
Air higroskopis adalah Land water and air pollution
Land water and air pollution Land water and air pollution
Land water and air pollution Aim or objectives of air pollution
Aim or objectives of air pollution Stationary and mobile sources of air pollution
Stationary and mobile sources of air pollution Baghouse filter definition apes
Baghouse filter definition apes Section 2 air noise and light pollution
Section 2 air noise and light pollution Section 2 air noise and light pollution
Section 2 air noise and light pollution Stock pollutants
Stock pollutants Definition of pollution in simple words
Definition of pollution in simple words The meaning of environmental pollution
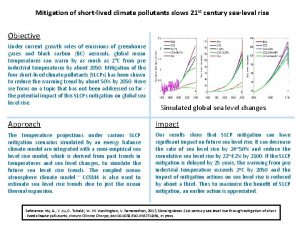
The meaning of environmental pollution Short lived climate pollutants
Short lived climate pollutants Why do thermal inversion layers trap pollutants
Why do thermal inversion layers trap pollutants Definition of pollution in simple words
Definition of pollution in simple words Introduction of pollution
Introduction of pollution Air pollution effect on plants
Air pollution effect on plants Air pollution contents
Air pollution contents Main cause of air pollution
Main cause of air pollution Air pollution box model example
Air pollution box model example General effects of air pollution
General effects of air pollution Air pollution consequences
Air pollution consequences Erg (air pollution control) ltd
Erg (air pollution control) ltd Air pollution
Air pollution Objectives of air pollution
Objectives of air pollution Air pollutant definition
Air pollutant definition Air pollution
Air pollution What is inorganic pollution
What is inorganic pollution Air pollution intro
Air pollution intro Two sources of air pollution
Two sources of air pollution 5 effects of air pollution
5 effects of air pollution Fixed box model air pollution
Fixed box model air pollution Northern sonoma county air pollution control district
Northern sonoma county air pollution control district Indoor air pollution examples
Indoor air pollution examples Air pollution simulator
Air pollution simulator Air pollution 2050
Air pollution 2050