Air Pollution Air Pollution Primary Pollutants l Come

























- Slides: 40

Air Pollution

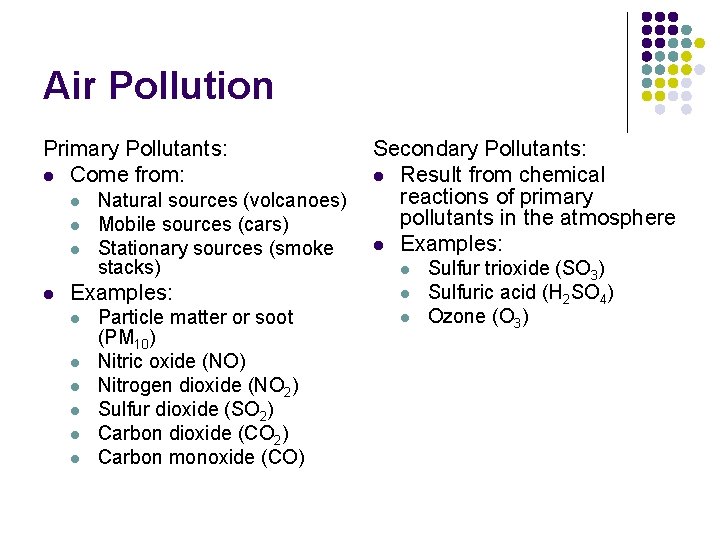
Air Pollution Primary Pollutants: l Come from: l l Natural sources (volcanoes) Mobile sources (cars) Stationary sources (smoke stacks) Examples: l l l Particle matter or soot (PM 10) Nitric oxide (NO) Nitrogen dioxide (NO 2) Sulfur dioxide (SO 2) Carbon dioxide (CO 2) Carbon monoxide (CO) Secondary Pollutants: l Result from chemical reactions of primary pollutants in the atmosphere l Examples: l l l Sulfur trioxide (SO 3) Sulfuric acid (H 2 SO 4) Ozone (O 3)

Major Air Pollutants l Criteria air pollutants l l l Set of pollutants that cause smog, acid rain, and other health hazards Emitted from industry, mining, transportation, power generation, and agriculture Include: ozone, particulate matter, carbon monoxide, sulfur dioxide, nitrogen oxides, and lead

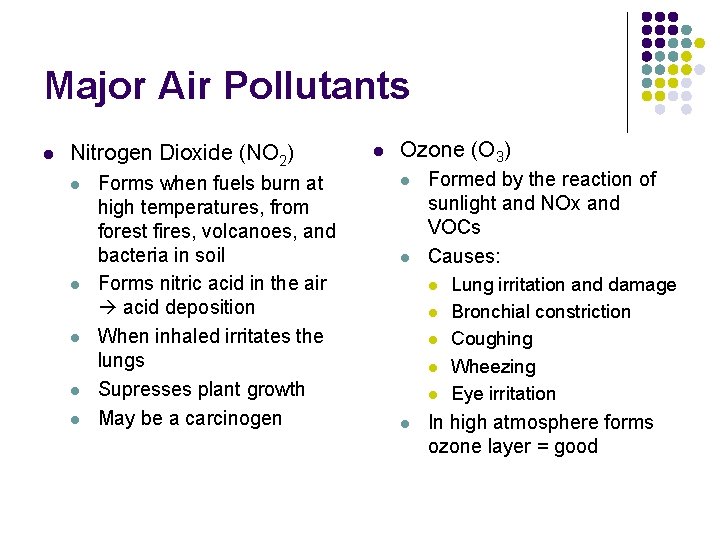
Major Air Pollutants l Nitrogen Dioxide (NO 2) l l l Forms when fuels burn at high temperatures, from forest fires, volcanoes, and bacteria in soil Forms nitric acid in the air acid deposition When inhaled irritates the lungs Supresses plant growth May be a carcinogen l Ozone (O 3) l l Formed by the reaction of sunlight and NOx and VOCs Causes: l l l Lung irritation and damage Bronchial constriction Coughing Wheezing Eye irritation In high atmosphere forms ozone layer = good

Major Air Pollutants l Peroxyacyl Nitrates (PANs) Hydrocarbons + O 2 +NO 2 + light CH 3 COOONO 2 (PAN) l. Stable in the atmosphere transport unstable compounds far away from urban centers l. Cause eye irritation l. In high concentrations damage vegetation l Sulfur Dioxide (SO 2) l l l Produced by burning highsulfur oil or coal, smelting of metals, and paper manufacturing Combines with water vapor to produce acid rain Acid rain causes: l l Reduction in plant productivity Breathing difficulties Destroys buildings Acidifies water supply

Major Air Pollutants l Suspended Particulate Matter (PM 10) l l l Particles with a diameter of 1/7 of a human hair or less Include smoke, dust, diesel soot, lead, and asbestos Cause lung irriation and damage Are mutagens, teratogens (interfers with development), and carcinogens Reducing PM 10 would produce health benefits 10 times grater than reducing all other air pollutants combined l Volatile Organic Compounds (VOCs) l l l Include organic compounds that have a high vapor pressure Found in paints, aerosol sprays, dry-cleaning fluids, and industrial solvents Cause respiratory irritation and damage Most are carcinogens Cause liver, kidney, and central nervous system damage May be greater concentrations indoors than outdoors

Measurement Units l ppm (Parts per million) l l 1 ppm means every 999, 999 particles of air there is 1 particle of pollutant Others: l l ppb or nano: parts per billion ppt or pico: parts per trillion

Smog l Industrial: tends to be sulfur based l l Called “grey-air” smog Photochemical: nitrogen based l l Catalyzed by UV radiation Called “brown-air” smog

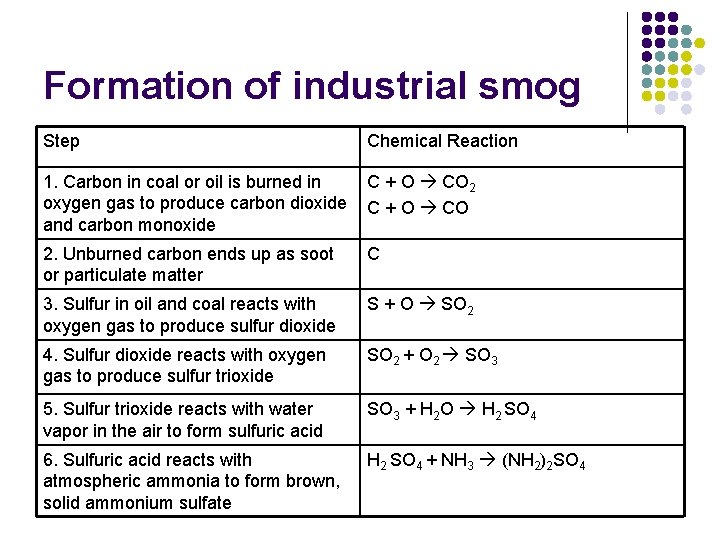
Formation of industrial smog Step Chemical Reaction 1. Carbon in coal or oil is burned in oxygen gas to produce carbon dioxide and carbon monoxide C + O CO 2. Unburned carbon ends up as soot or particulate matter C 3. Sulfur in oil and coal reacts with oxygen gas to produce sulfur dioxide S + O SO 2 4. Sulfur dioxide reacts with oxygen gas to produce sulfur trioxide SO 2 + O 2 SO 3 5. Sulfur trioxide reacts with water vapor in the air to form sulfuric acid SO 3 + H 2 O H 2 SO 4 6. Sulfuric acid reacts with atmospheric ammonia to form brown, solid ammonium sulfate H 2 SO 4 + NH 3 (NH 2)2 SO 4

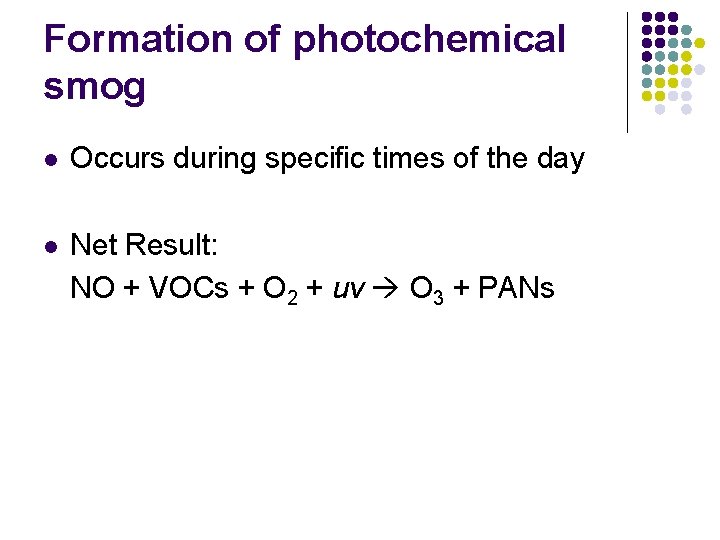
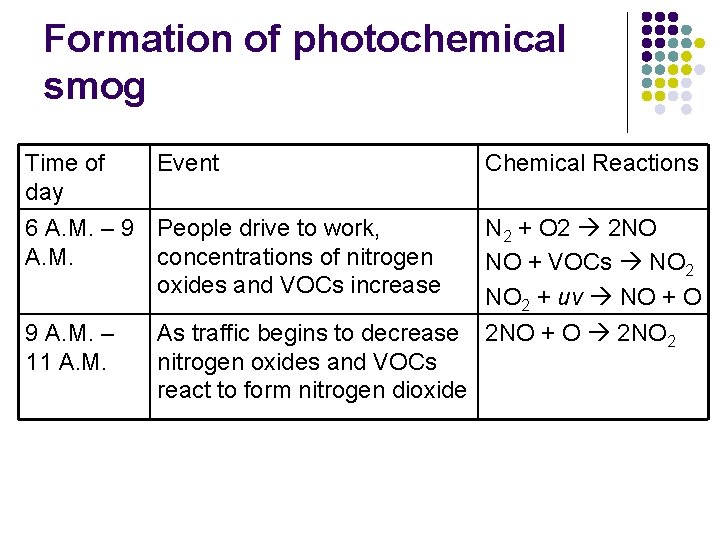
Formation of photochemical smog l Occurs during specific times of the day l Net Result: NO + VOCs + O 2 + uv O 3 + PANs

Formation of photochemical smog Time of day Event 6 A. M. – 9 People drive to work, A. M. concentrations of nitrogen oxides and VOCs increase 9 A. M. – 11 A. M. Chemical Reactions N 2 + O 2 2 NO NO + VOCs NO 2 + uv NO + O As traffic begins to decrease 2 NO + O 2 NO 2 nitrogen oxides and VOCs react to form nitrogen dioxide

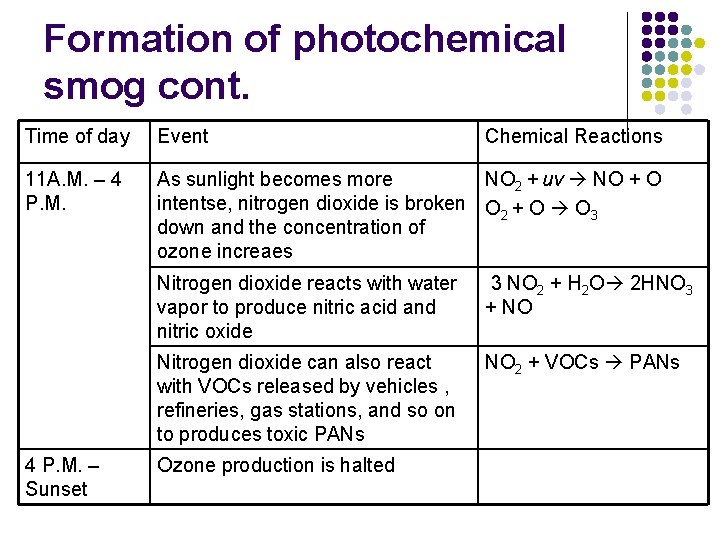
Formation of photochemical smog cont. Time of day Event 11 A. M. – 4 P. M. NO 2 + uv NO + O As sunlight becomes more intentse, nitrogen dioxide is broken O 2 + O O 3 down and the concentration of ozone increaes 4 P. M. – Sunset Chemical Reactions Nitrogen dioxide reacts with water vapor to produce nitric acid and nitric oxide 3 NO 2 + H 2 O 2 HNO 3 + NO Nitrogen dioxide can also react with VOCs released by vehicles , refineries, gas stations, and so on to produces toxic PANs NO 2 + VOCs PANs Ozone production is halted

Case Study – Great Smog of ‘ 52 l l A period of cold weather combined with windless conditions to collect airborne pollutants (most form coal) to produce a thick layer of grey smog over London December 5 – 9 1952 Dispersed quickly after a change in weather 100, 000 people became ill 12, 000 died

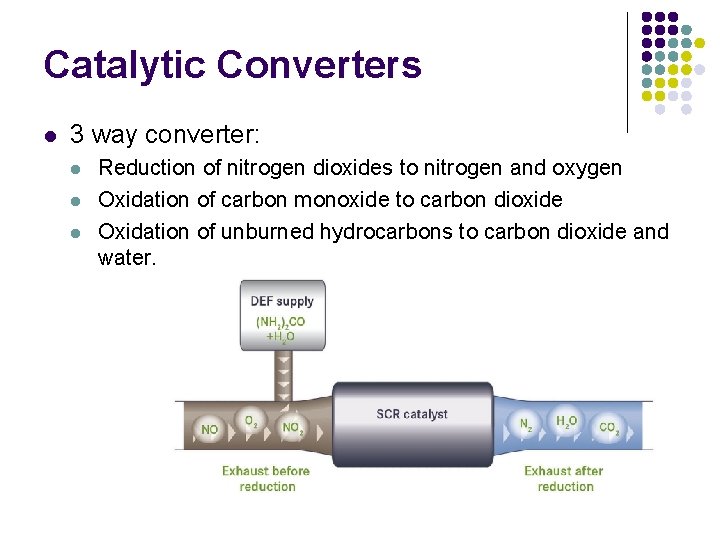
Catalytic Converters l l Converts toxic chemicals in engine exhaust to less noxious substances Inside a catalyst stimulates a chemical reaction in which noxious by-products of combustion are converted to less toxic substances

Catalytic Converters l 3 way converter: l l l Reduction of nitrogen dioxides to nitrogen and oxygen Oxidation of carbon monoxide to carbon dioxide Oxidation of unburned hydrocarbons to carbon dioxide and water.

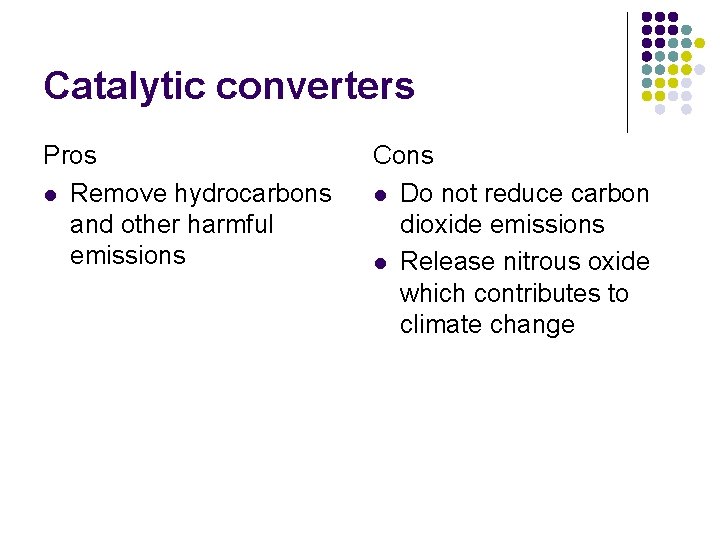
Catalytic converters Pros l Remove hydrocarbons and other harmful emissions Cons l Do not reduce carbon dioxide emissions l Release nitrous oxide which contributes to climate change

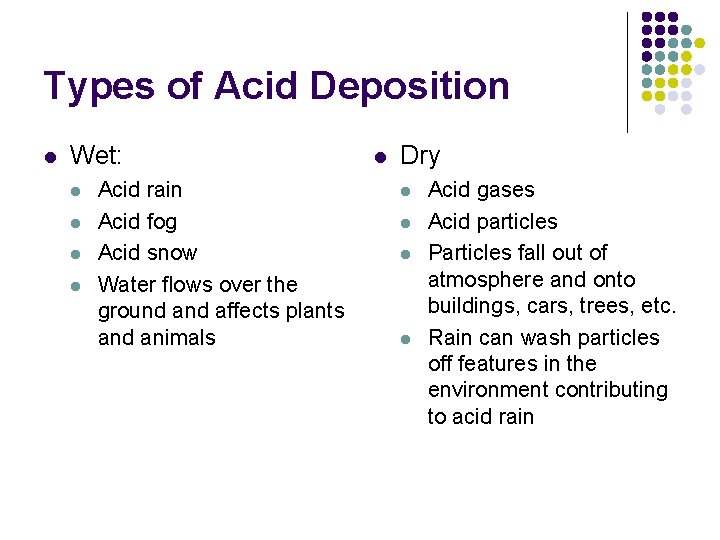
Types of Acid Deposition l Wet: l l Acid rain Acid fog Acid snow Water flows over the ground affects plants and animals l Dry l l Acid gases Acid particles Particles fall out of atmosphere and onto buildings, cars, trees, etc. Rain can wash particles off features in the environment contributing to acid rain

Acid depositions l Due to: l l l Sulfur dioxide Nitrogen oxides Environmental effects: l l Acidify streams Damage forests soils through nitrogen saturation Acid shock (rapid melting of snow pack with acid particles) Leaches essential plant nutrients from the soil

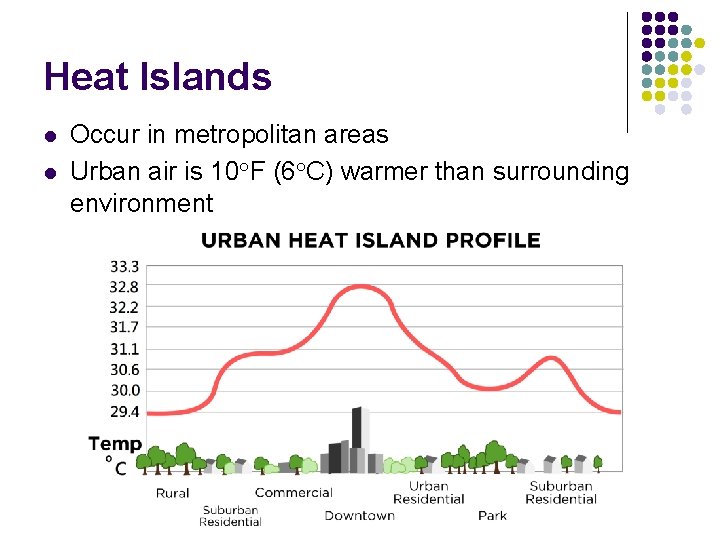
Heat Islands l l Occur in metropolitan areas Urban air is 10°F (6°C) warmer than surrounding environment

Heat Islands l Causes: l l Buildings reduce radiation of heat to the atmosphere Thermal properties of surface material (asphalt, bricks, concrete) store heat longer Lack of vegetation and standing water increase temperatures Human activities (automobiles, industry, etc. ) l Effects: l l l Combined with high levels of pollution leads to a localized green house effect Excessive temperatures can lead to deaths Meteorological effects: l Alter local wind patterns l Alter the development of clouds and fog l Alter number of lighting strikes l Change precipitation patterns

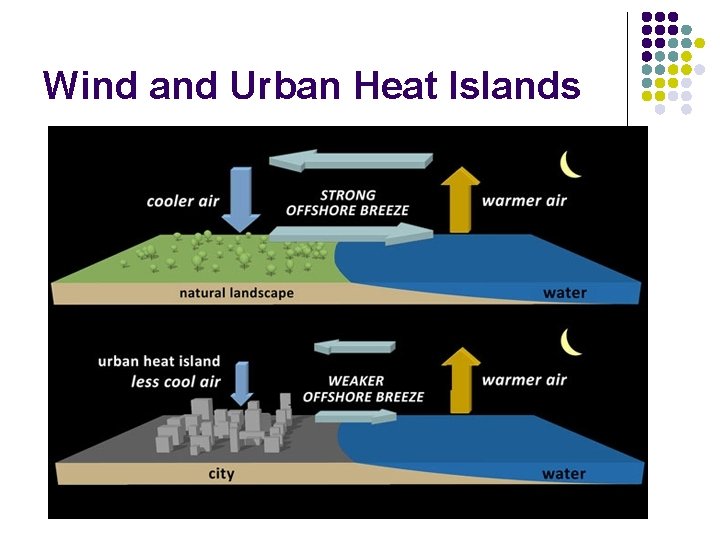
Wind and Urban Heat Islands

Combating urban heat islands l l Increasing amount of landscaping in parks and on top of buildings Increasing the amount of light or reflective material

Smart Cities

Temperature Inversions l Occur when air temperature increases with height above the ground l l Usually occur at night when the surface cools, cooling the air above it Can lead to smog being trapped near the ground human health problems (asthma, emphysema, and increase in lung cancer)

Case Study- Donora, PA 1948 l l Smog from the local zinc and steel smelting plants settled in the valley where Donora was located 20 people asphyxiated and 7, 000 went to the hospital (total population of the town was 14, 000) Four days later, wind cleared the toxins from the town Led to first meaningful federal and state laws to control air pollution

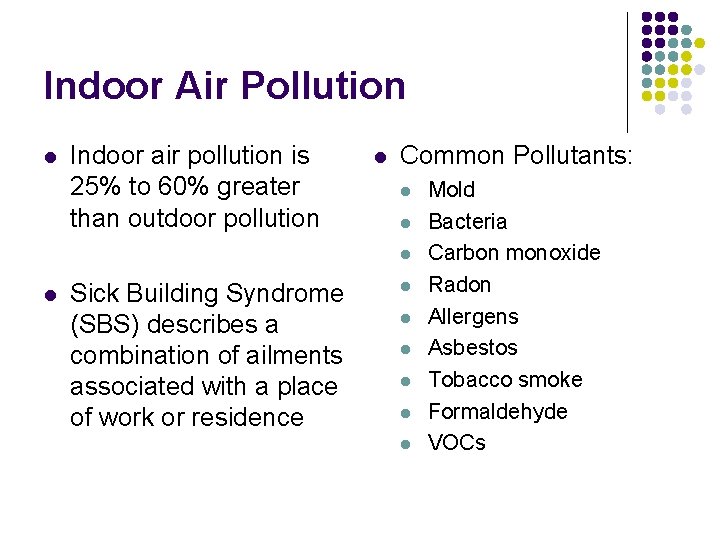
Indoor Air Pollution l Indoor air pollution is 25% to 60% greater than outdoor pollution l Common Pollutants: l l Sick Building Syndrome (SBS) describes a combination of ailments associated with a place of work or residence l l l Mold Bacteria Carbon monoxide Radon Allergens Asbestos Tobacco smoke Formaldehyde VOCs

SBS symptoms l l l l Headaches Breathing difficulties Allergies Asthma Cancer Emphysema Nerve disorders

Air Pollution Remediation and Reduction Strategies 1. 2. 3. 4. 5. Emphasizing tax incentives for pollution control rather than fines and penalties Setting legislative standards for energy efficiency Increasing funding for research into renewable energy resources Incorporating incentives for reducing air pollution into trade policies Distributing solar cook stoves to developing countries to replace coal and firewood

Air Pollution Remediation and Reduction Strategies Phasing out two-cycle gasoline engines For issues involving SBS 6. 7. a. b. c. d. e. 8. Modify building codes to control materials used in construction Replace and repair areas that have received water damage to control for mold Use paints, adhesives, solvents, cleaning products, and pesticides in well-ventilated areas and during nonoccupancy Increase the number of complete air exchanges in buildings Ensure proper maintenance of HVAC systems Providing incentives to use mass transit

Combating Acid Rain l l l Design more efficient engines to reduce emissions Reduce emissions in coal-burning power plants Increase penalties on stationary sources Provide incentives to consumers to purchase Energy Star products Increase CAFE standards

EPA’s Acid Rain Program l l Designed to achieve significant environmental reduction and public health benefits through reductions in emission of sulfur and nitrogen compounds at a low cost to society Encourages energy efficiency and pollution prevention l Strategies: l l l Allowance trading system Opt-in program, allows nonaffected industrial and small utility units to participate in trading Setting new NOx emission standards Permit process that affords flexibility in selecting most cost-effective approach Continuous Emissions Monitoring (CEM) provide accounting of excess emissions

Clean Air Act (1963) l l Designed to control pollution on a national level Act has been amended in 1967, 1977, 1990 Requires EPA to design and enforce regulations to protect the public from airborne contaminants Required comprehensive federal and state regulations for both stationary and mobile sources of pollution l l Expanded federal enforcement authority Addressed acid rain, ozone depletion, and toxic air pollution Established new gasoline reformulation requirements 1 st major environmental law to include provisions for citizen law suits

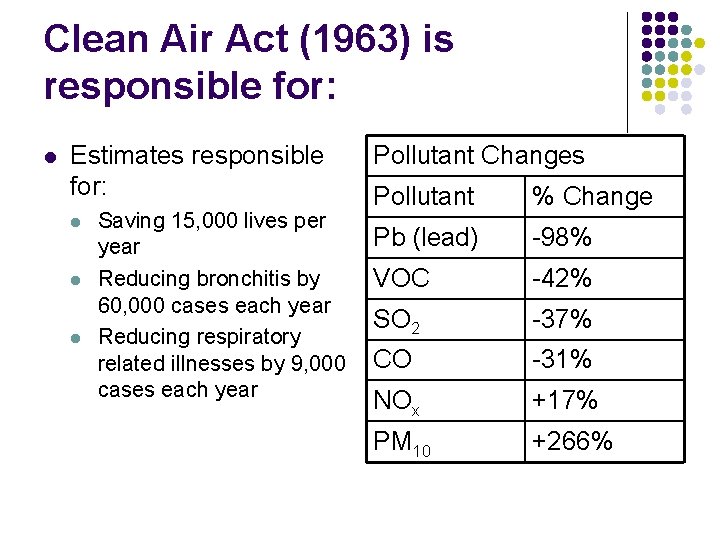
Clean Air Act (1963) is responsible for: l Estimates responsible for: l l l Saving 15, 000 lives per year Reducing bronchitis by 60, 000 cases each year Reducing respiratory related illnesses by 9, 000 cases each year Pollutant Changes Pollutant % Change Pb (lead) -98% VOC -42% SO 2 -37% CO -31% NOx +17% PM 10 +266%

Relevant laws l Air Pollution Control Act (1955) l l l 1 st legislation regarding air pollution Identified air pollution as a national problem Announced research and additional steps were necessary Basically meant to make public aware of problem National Environmental Policy Act (1970) l l Created EPA Mandated the creation of Environmental Impact Statements

Relevant laws l Montreal Protocol (1989) l l Agreement among nations to phase out chemicals that damage the ozone layer Pollution Prevention Act (1990) l l Requires industry to reduce pollution at its source Reduction can be in terms of volume and/or toxicity

Kyoto Protocol (1997 and 2001) l l An agreement among 150 nations requiring greenhouse gas reductions Would have required a 7% decrease in greenhouse gas emissions compare to 1990 levels over a 5 year period l l l US felt protocol held developed and developing countries to different standards Concerned the costs were too high and time frame too short; climate change deniers 2012: Canada, Japan, and Russia joined the US saying they would not sign an agreement unless unbalanced requirements for developed and developing countries were changed

Noise Pollution l Unwanted human-created sound that disrupts the environment l l l Transportation noise (dominant) Office equipment Factory machinery Appliances Power tools Audio entertainment systems

Noise Pollution Effects l Short Term l l l Damage to inner ear results in hearing loss Cardiovascular problems Gastric-intestinal problems Decrease alertness and ability to memorize Nervousness, pupil dilation Decrease in the visual field l Long term l l l l Insomnia Nervousness Bulimia chronically high blood pressure Anxiety Depression Sexual dysfunction

Noise Pollution Control Measures l Road noise: l l l Use of noise barriers, limitations on vehicle speed newer roadway surface technologies traffic control limiting times for heavy duty vehicles Air line Noise: l l Developing quieter jet engines controlling take off and landing times l Industrial Noise: l l l New technologies Instillations of noise barriers in the work place Residential Noise: l Regulate noise from power tools, garden equipement, loud radios

Relevant Law l Noise Pollution Control Act (1972): l l Establishes a national policy to promote an environment free from noise that jeopardizes health Establishes a means for the coordination of federal research activities in noise control Authorizes establishment of federal noise standards and products Provides information to the public respecting noise emission and noise reduction characteristics of such protocol
 Trees
Trees Primary pollutants and secondary pollutants
Primary pollutants and secondary pollutants What are the secondary air pollutants
What are the secondary air pollutants What are secondary pollutants
What are secondary pollutants Primary vs secondary pollutants
Primary vs secondary pollutants Differentiate between primary and secondary pollutants
Differentiate between primary and secondary pollutants Primary vs secondary pollutants
Primary vs secondary pollutants Primary and secondary pollutants difference
Primary and secondary pollutants difference Come rico come sano
Come rico come sano Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Secondary air pollutants
Secondary air pollutants Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air What are the secondary air pollutants
What are the secondary air pollutants Major air pollutants
Major air pollutants Air pollutants
Air pollutants Indoor air pollution sources
Indoor air pollution sources Chapter 12 section 1 what causes air pollution
Chapter 12 section 1 what causes air pollution Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Secondary pollutants examples
Secondary pollutants examples Stock pollutants
Stock pollutants Environmental pollution meaning
Environmental pollution meaning Environmental pollution definition
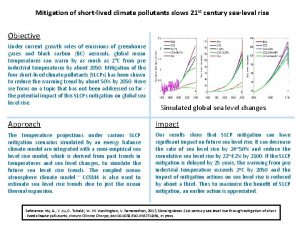
Environmental pollution definition Short lived climate pollutants
Short lived climate pollutants Solar energy and the atmosphere
Solar energy and the atmosphere Hubungan air tanah dan tanaman
Hubungan air tanah dan tanaman Come holy spirit dove divine
Come holy spirit dove divine He-y, come on ou-t!
He-y, come on ou-t! Come thou fount come thou king lyrics
Come thou fount come thou king lyrics Past participle
Past participle Emmanuel son of god
Emmanuel son of god Come mi chiamo
Come mi chiamo Through all generations everlasting is his mercy
Through all generations everlasting is his mercy Softly and tenderly jesus is calling
Softly and tenderly jesus is calling Come mi vedo io
Come mi vedo io Come on come on turn your radio on
Come on come on turn your radio on Come mi chiamo come mi chiamo
Come mi chiamo come mi chiamo Drink in participle
Drink in participle Come in come in and sit down
Come in come in and sit down Come in come in and sit down
Come in come in and sit down The primary pigments are _____ the primary colors.
The primary pigments are _____ the primary colors. Effects of land pollution on human health
Effects of land pollution on human health