Adsorption Theory of Heterogenous catalysis Explains mechanism Old


- Slides: 15

Adsorption Theory of Heterogenous catalysis

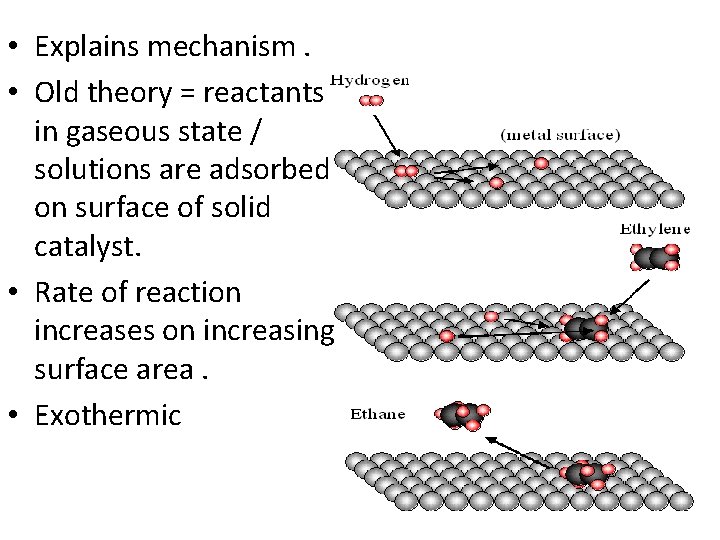
• Explains mechanism. • Old theory = reactants in gaseous state / solutions are adsorbed on surface of solid catalyst. • Rate of reaction increases on increasing surface area. • Exothermic

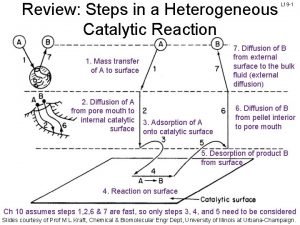
• Modern Theory-combination of intermediate compound theory and & old adsorbtion theory. • Steps – • 1. Diffusion • 2. Adsorption • 3. Formation of intermediate. • 4. Desorption • 5. Diffusion

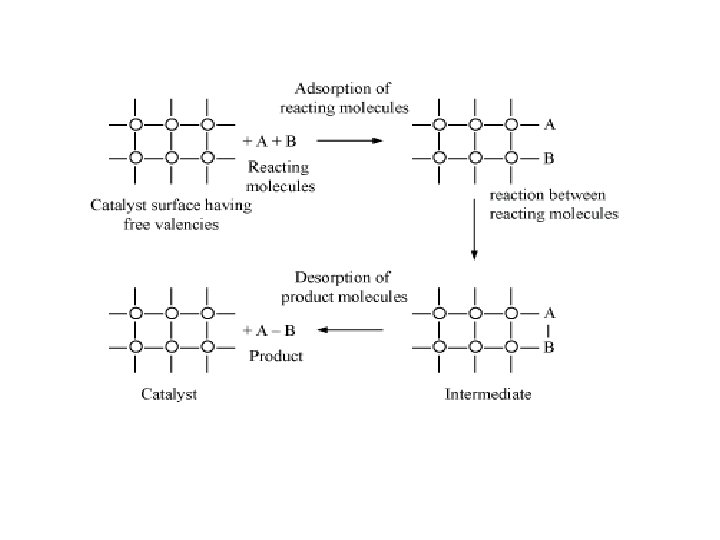
• Free valencies present on surface catalysts. • Provides seats for chemical raction. • When gas molecules comes in contact , they held up due to loose combination. • • Side by side adsorbtion results in formation of new molecule.


Important features of solid catalysts • Activity –. Depends upon the strength of chemisorption to a large extent. Reactant must get adsorbed on catalyst to become active. Catalytic activity –maximum in Gp. 7 to Gp 9 metals

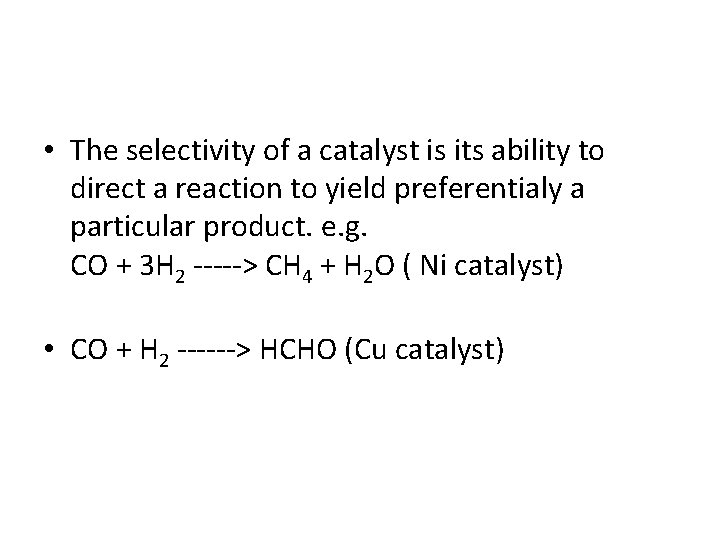
• The selectivity of a catalyst is its ability to direct a reaction to yield preferentialy a particular product. e. g. CO + 3 H 2 -----> CH 4 + H 2 O ( Ni catalyst) • CO + H 2 ------> HCHO (Cu catalyst)

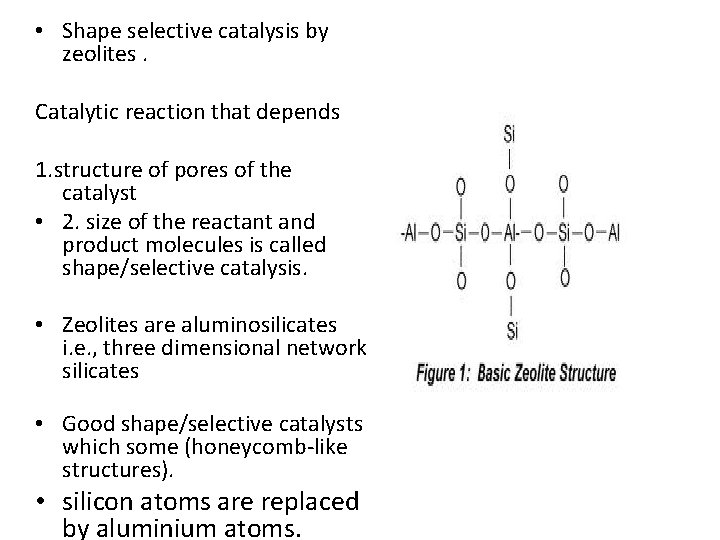
• Shape selective catalysis by zeolites. Catalytic reaction that depends 1. structure of pores of the catalyst • 2. size of the reactant and product molecules is called shape/selective catalysis. • Zeolites are aluminosilicates i. e. , three dimensional network silicates • Good shape/selective catalysts which some (honeycomb-like structures). • silicon atoms are replaced by aluminium atoms.

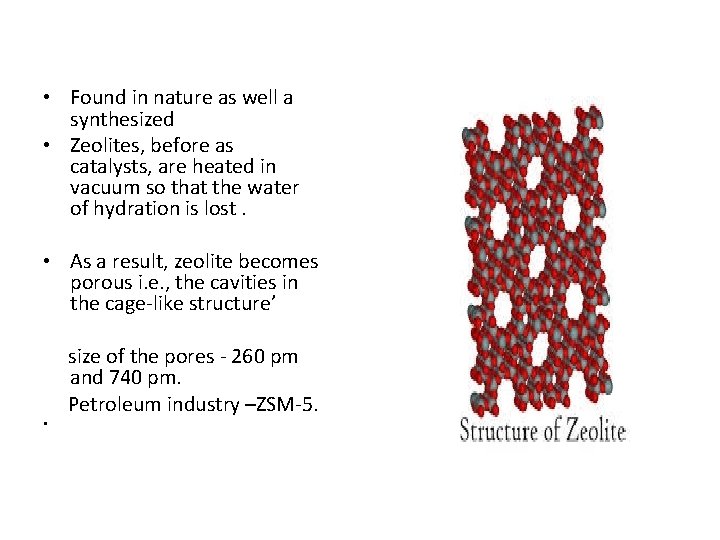
• Found in nature as well a synthesized • Zeolites, before as catalysts, are heated in vacuum so that the water of hydration is lost. • As a result, zeolite becomes porous i. e. , the cavities in the cage-like structure’ • size of the pores - 260 pm and 740 pm. Petroleum industry –ZSM-5.

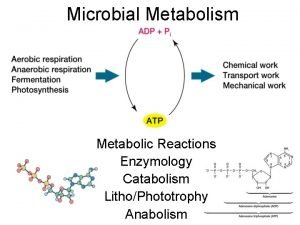
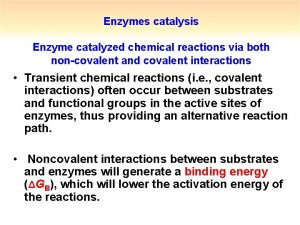
Enzyme catalysis • • Complex nitrogeneous organic compounds. Sources –Plants & animals. Protein molecules of high molecular mass. Forms colloidal solutions in water. Effective catalysts. Biological catalysts. First enzyme-synthesised in 1969.

• Examples • Inversion of cane sugar. • Conversion of glucose into ethyl alcohol.

Characteristics of enzyme catalysis 1. Hihly efficient 2. Highly specific nature 3. Highly active under optimum T(298 -310) 4. Highly active under optimum p. H(5 -7) 5. Increasing activity in presence of activators & coenzymes –(non protein +enzymes) • 6. Influence of inhibitors &poisons. • • •

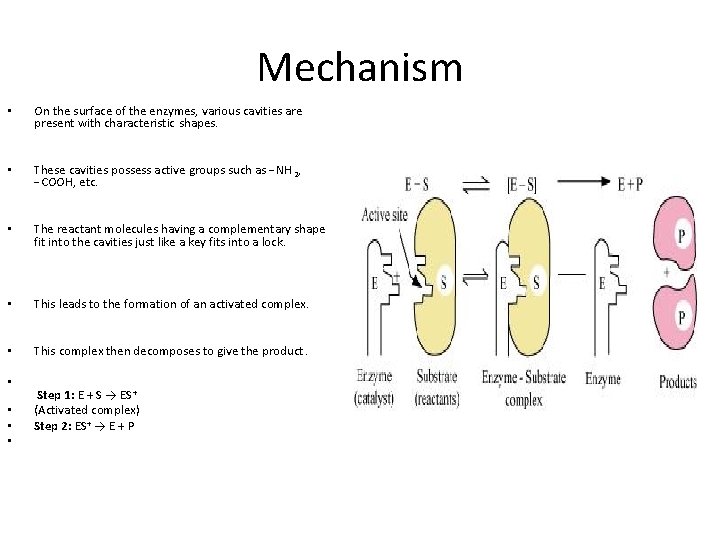
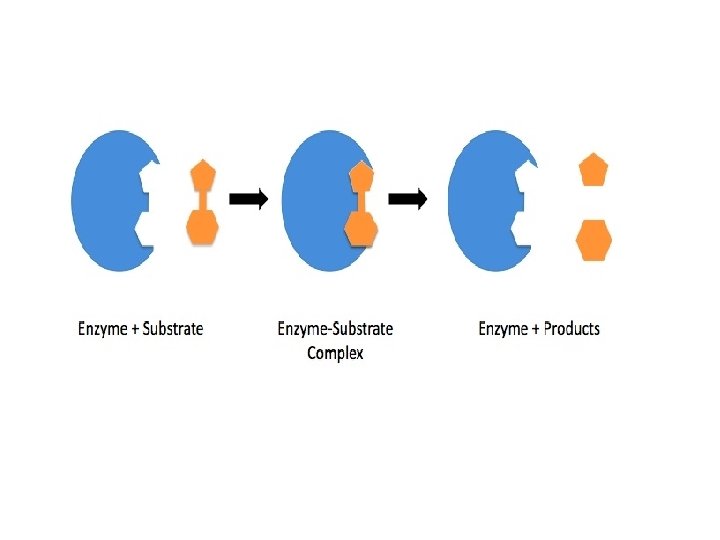
Mechanism • On the surface of the enzymes, various cavities are present with characteristic shapes. • These cavities possess active groups such as −NH 2, −COOH, etc. • The reactant molecules having a complementary shape fit into the cavities just like a key fits into a lock. • This leads to the formation of an activated complex. • This complex then decomposes to give the product. • • Step 1: E + S → ES + (Activated complex) Step 2: ES+ → E + P


Catalysts in industry • Haber’s process –Ammonia • Conditions –Finely divided Fe, Mo/200 bar/723773 K • Ostwalds process –Nitric acid • Conditions-Platinised aesbestos /573 K • Contact process • Condition-V 2 O 5/pt aesbestos /673 -723
 Langmuir-hinshelwood mechanism heterogeneous catalysis
Langmuir-hinshelwood mechanism heterogeneous catalysis Mechanism of chromatography
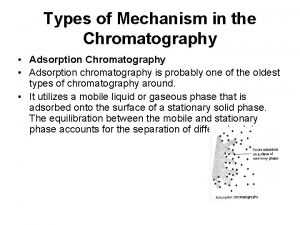
Mechanism of chromatography _____ solutions are very unstable.
_____ solutions are very unstable. Heterogenous team
Heterogenous team Heterogeneous audience definition
Heterogeneous audience definition Heterogeneous class
Heterogeneous class Heterogenous
Heterogenous Solutions are heterogenous mixtures
Solutions are heterogenous mixtures Dry gum method is also known as
Dry gum method is also known as 7 steps of heterogeneous catalysis
7 steps of heterogeneous catalysis Enzymatic catalysis
Enzymatic catalysis Catalysis by approximation
Catalysis by approximation What is covalent catalysis
What is covalent catalysis What is covalent catalysis
What is covalent catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis