Accidents Authorization and Inspection of Radiation Sources in
















- Slides: 32

Accidents

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Objective To understand the consequences of accidents in nuclear medicine. 2

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Contents • Deviation from prescribed dose; • Accidental medical exposure; • Case studies and lessons learned. 3

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Accident An accident is “any unintended event, including operating errors, equipment failures or other mishaps, the consequences or potential consequences of which are not negligible from the point of view of protection and safety. ” [GSR Part 3 Definitions] 4

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Accidental Medical Exposure In the event of an accident: “Registrants and licensees shall promptly investigate any of the following unintended or accidental medical exposures: (a) any therapeutic treatment delivered to either the wrong patient or the wrong tissue, or using the wrong pharmaceutical, or with a dose or dose fractionation differing substantially from the values prescribed by the medical practitioner or which may lead to undue acute secondary effects; ” [GSR Part 3 Requirement 41. 3. 179] 5

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Accidental Medical Exposure (cont) In the event of an accident the licensee shall take action to: • estimate the dose received; • take measures to prevent a reoccurrence; • notify the Regulatory Body; • inform the patient and their doctor. 6

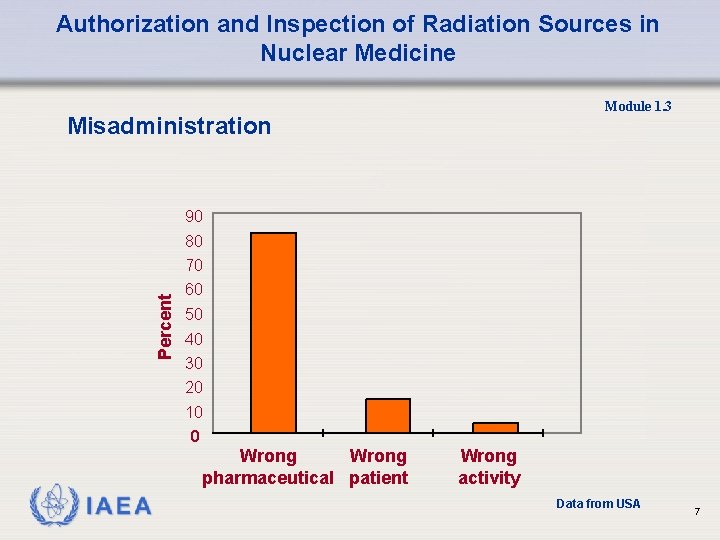
Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration 90 80 Percent 70 60 50 40 30 20 10 0 Wrong pharmaceutical patient Wrong activity Data from USA 7

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Wrong Radiopharmaceutical • wrong patient; • wrong route of administration; • wrong activity: q therapy >10% from prescribed activity. q diagnosis > 50% from prescribed activity. 8

Authorization and Inspection of Radiation Sources in Nuclear Medicine Misadministration – Wrong Patient • Module 1. 3 A therapy dose of 350 MBq 131 I was administered to patient A instead of patient B. Patient A had been prescribed 500 MBq 99 m. Tc for a bone scan. The 99 m. Tc was administered to A and the patient seated in the waiting room. • Patient B, who was scheduled for the 131 I treatment arrived later, completed an interview and was seated in the waiting room. The technologist prepared the 131 I and called patient B but patient A responded. • The technologist explained the treatment, scheduled a follow-up appointment and administered the activity. The patient then questioned the procedure and it became clear that the wrong patient had received the 131 I. 9

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Wrong Patient (cont) • • Patient A was immediately informed of the error and his stomach was pumped, retrieving about 1/3 rd the activity. The patient was administered perchlorate and Lugol’s solution to block further uptake by the thyroid. The misadministration resulted in an absorbed dose to the thyroid of the wrong patient (A) of about 8 Gy. Initiating event: A patient responded to another patient’s name. Contributing factor: Hospital protocol for identification of patients was not followed 10

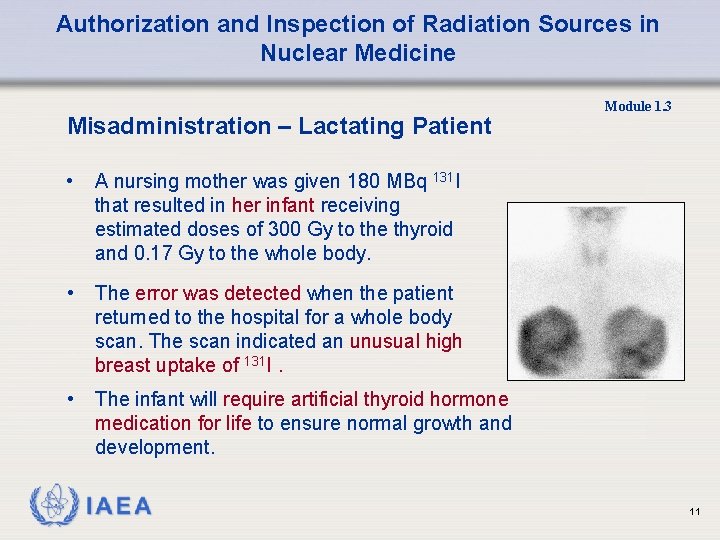
Authorization and Inspection of Radiation Sources in Nuclear Medicine Misadministration – Lactating Patient • A nursing mother was given 180 MBq 131 I that resulted in her infant receiving estimated doses of 300 Gy to the thyroid and 0. 17 Gy to the whole body. • The error was detected when the patient returned to the hospital for a whole body scan. The scan indicated an unusual high breast uptake of 131 I. • The infant will require artificial thyroid hormone medication for life to ensure normal growth and development. Module 1. 3 11

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Lactating Patient (cont) Contributing factor: The technologist was distracted and forgot to ask a standard list of questions Initiating event A dose of 131 I was given to a nursing mother 12

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Pregnant Patient A 43 year old female patient was scheduled for a thyroid scan. • She called the department in the morning and told the technologist that she was trying to get pregnant but there was no evidence at the moment that she was. • The technologist misunderstood the patient and she was persuaded to undertake the examination. • Later, it appeared that the patient was in the very early stages of pregnancy and subsequently had a miscarriage. 13

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Pregnant Patient (cont) Contributing factor • Communication failure. • Inappropriate local rules. Initiating event Examination of a pregnant woman. 14

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Wrong route A technologist cursorily read an examination request form noting that it involved 99 m. Tc DTPA. • A standard dose of the radiopharmaceutical was prepared and injected before it was noted that the requested procedure required inhalation of the radiopharmaceutical in aerosol form. 15

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Wrong route (cont) Initiating event Wrong route of administration Contributing factor Failed to carefully read the request form 16

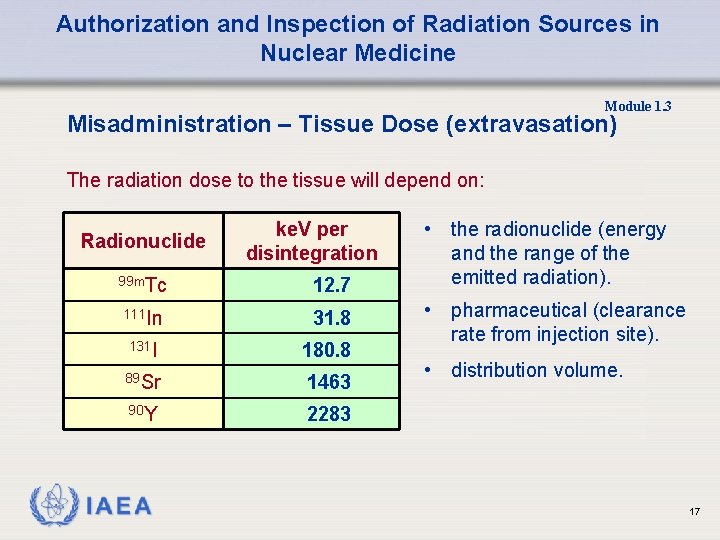
Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Tissue Dose (extravasation) The radiation dose to the tissue will depend on: Radionuclide ke. V per disintegration 99 m. Tc 12. 7 111 In 31. 8 131 I 180. 8 89 Sr 1463 90 Y 2283 • the radionuclide (energy and the range of the emitted radiation). • pharmaceutical (clearance rate from injection site). • distribution volume. 17

Authorization and Inspection of Radiation Sources in Nuclear Medicine Misadministration – Wrong Activity Module 1. 3 A patient was to be administered 259 MBq 131 I. • The radiopharmaceutical was in two 130 MBq capsules and was so indicated on the vial label. • Previous doses at the hospital had been administered in the form of one 259 MBq capsule. • When the vial was inverted one of the two capsules fell out and the technologist assumed this was the entire dose. • Much later the other capsule was detected. The patient received only 50% of the prescribed activity. 18

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Wrong Activity (cont) Contributing factor • Absence of cross check of the vial label with respect to both activity and number of capsules. • No measurement of the activity before treatment. Initiating event One of two capsules remained stuck in the vial 19

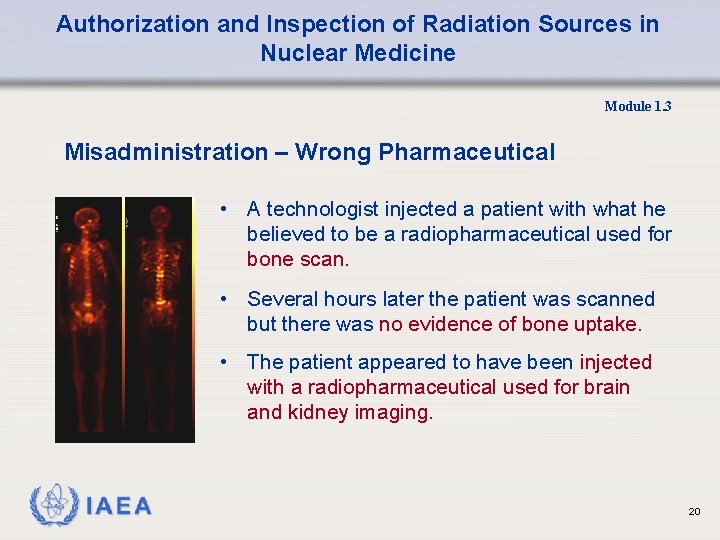
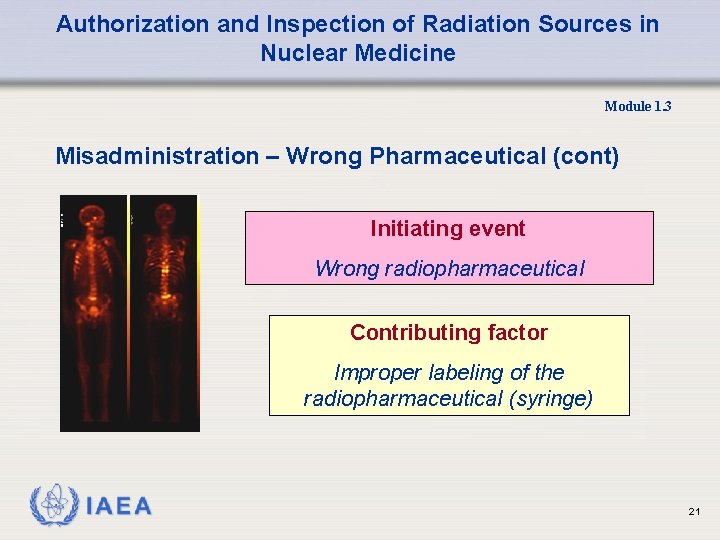
Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Wrong Pharmaceutical • A technologist injected a patient with what he believed to be a radiopharmaceutical used for bone scan. • Several hours later the patient was scanned but there was no evidence of bone uptake. • The patient appeared to have been injected with a radiopharmaceutical used for brain and kidney imaging. 20

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration – Wrong Pharmaceutical (cont) Initiating event Wrong radiopharmaceutical Contributing factor Improper labeling of the radiopharmaceutical (syringe) 21

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration Consequences • Non-justified exposure • Increased radiation risks • Delayed diagnosis • Increased costs • Increased workload • Reduced confidence 22

Authorization and Inspection of Radiation Sources in Nuclear Medicine Misadministration counter measures Module 1. 3 Immediately use all available means to minimize any adverse effects, such as: • • expeditious removal of orally administered radiopharmaceuticals by emesis, gastric lavage, laxatives or enemas. accelerated excretion of intravenously administered radiopharmaceuticals by hydration, diuresis, etc. • removal of urine by catheterization from patients who cannot void spontaneously. • when appropriate, use of blocking agents to diminish the absorbed dose to the thyroid gland, salivary glands and stomach. 23

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Misadministration causes • Communication problems. • Busy environment, distraction. • Unknown local rules. • No training in emergency situations. • Not clearly defined responsibilities. • No efficient Quality Assurance. 24

Authorization and Inspection of Radiation Sources in Nuclear Medicine Avoiding Accidents and Misadministrations Module 1. 3 • Safety culture. • Safety assessment to define critical procedures and emergency situations. • Reporting system (When? Where? Why? ). • Education and training: q initial; q continuing. 25

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Investigating accidental medical exposures • Inform the responsible nuclear medicine physician. • Inform the patient and the referring physician. • Calculate the dose. • Indicate the corrective measures to be taken. • Implement those measures. • Submit report to the licensee’s Radiation Protection Committee and to the Regulatory Body. 26

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Lessons Learned • Communication errors. • Errors in patient identification. • Using the wrong radiopharmaceutical or the wrong activity. • Calibration errors and/or maintenance failure. Studies have shown that most accidents could have been prevented by consistent application of the Requirements 34 – 42 (3. 144 – 3. 184) of the GSR Part 3. 27

Authorization and Inspection of Radiation Sources in Nuclear Medicine Accidents and Lessons Learned - Example Module 1. 3 An 87 year old patient was administered 7. 4 GBq 131 I to relieve esophageal compression caused by metastatic thyroid carcinoma. • About 34 hours after receiving the dose, the patient had a cardiopulmonary arrest and died. Attempts at resuscitation were made in the patient’s room by 16 staff members. The efforts included insertion of a pacemaker. • Contaminated blood and urine were spilled and no surveys of the clothing of those present were done. The highest dose recorded was 0. 3 m. Gy for one nurse. • Even though the contamination was extensive, subsequent thyroid uptake measurements showed no uptakes by involved staff. 28

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Accidents and Lessons Learned – Example (cont) Initiating event Heart failure of patient shortly after iodine therapy Contributing factor • Contingency procedures for emergency situations involving radionuclides were not available. • Monitoring instruments and decontamination equipment were not available. No simulation exercises had been performed. 29

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Medical Emergency Contact the RPO for specific instructions. Medical personnel should proceed with emergency care while attempting to take precautions against spread of contamination but: • avoid direct contact with the patient’s mouth, • all members of the emergency team should wear impermeable protective gloves. Medical personnel shall be informed and trained in procedures for dealing with a radioactive patient. 30

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 Medical Emergency (cont) Radiation protection considerations should not prevent or delay life -saving operations in the event surgery on the patient is required. However, the following precautions should be observed: • Notify the operating room staff. • Modify operating procedures under the supervision of RPO to minimize exposure and spread of contamination. • Protective equipment may be used as long as efficiency and speed is not affected. • Rotation of personnel may be necessary if the surgical procedure is lengthy. The RPO should monitor individual doses to members of the staff. 31

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 1. 3 References • Manual on Radiation Protection in Hospitals and General Practice, Volume 4, Nuclear Medicine. IAEA/WHO. • Manual on therapeutic use of Iodine-131. Practical Radiation Safety Guide, IAEA. 32
 Print and web sources
Print and web sources Ionizing radiation sources
Ionizing radiation sources Ionizing radiation sources
Ionizing radiation sources Sources of radiation
Sources of radiation Important water resources
Important water resources Chapter 14:1 using body mechanics
Chapter 14:1 using body mechanics Chapter 13:2 preventing accidents and injuries
Chapter 13:2 preventing accidents and injuries Asp.net mvc 5 identity authentication and authorization
Asp.net mvc 5 identity authentication and authorization Ohsu health plan
Ohsu health plan Authentication authorization auditing
Authentication authorization auditing Grid security infrastructure
Grid security infrastructure Goal freedom alertness theory
Goal freedom alertness theory Law of conservation of momentum
Law of conservation of momentum Texte argumentatif sur les accidents de la route
Texte argumentatif sur les accidents de la route 7 types of kitchen accidents
7 types of kitchen accidents Most accidents are caused by _________ driving error.
Most accidents are caused by _________ driving error. Chapter 20 preventing kitchen accidents
Chapter 20 preventing kitchen accidents Preventing kitchen accidents worksheet
Preventing kitchen accidents worksheet Accidents sportifs statistiques
Accidents sportifs statistiques Scissor aerial work platform
Scissor aerial work platform Accidents/illness of family members
Accidents/illness of family members Av accidents
Av accidents Industrial radiography accidents
Industrial radiography accidents Theatre accidents
Theatre accidents Take away any liquid near your working area
Take away any liquid near your working area Pyramide de bird pour les nuls
Pyramide de bird pour les nuls Statistiques accidents du travail d'origine électrique
Statistiques accidents du travail d'origine électrique Industrial radiography accidents
Industrial radiography accidents Accidents in construction industry
Accidents in construction industry Accidents gramaticals
Accidents gramaticals Accidents & disasters
Accidents & disasters Accidents & disasters
Accidents & disasters An insurance company checks police records on 582 accidents
An insurance company checks police records on 582 accidents