Radiopharmaceuticals Authorization and Inspection of Radiation Sources in












- Slides: 21

Radiopharmaceuticals

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Objective To become familiar with the production and utilization of radiopharmaceuticals used in nuclear medicine. 2

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Contents • Production of radionuclides. • 99 Mo / 99 m. Tc generators. • Preparation of radiopharmaceuticals. • Good manufacturing practice. 3

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 4

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Commonly used Radiopharmaceuticals • The primary radionuclide used for diagnostic nuclear medicine procedures is Technetium-99 m (99 m. Tc). • The primary radionuclide used for therapeutic nuclear medicine procedures is Iodine-131 (131 I). 5

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Radiopharmaceutical Generators Radiopharmaceutical generators: • are constructed on the principle of the decay-growth relationship between a long-lived parent and its short-lived daughter radionuclide. q i. e. a long-lived parent nuclide is allowed to decay to its short-lived daughter nuclide and the latter is then chemically separated. e. g. 99 Mo (T½ = 66. 6 hours) 99 m. Tc (T½ = 6 hours) 6

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Radiopharmaceutical Generators (cont) The importance of generators lies in the fact that they: • are easily transportable; and • can serve as sources of short-lived radionuclides in institutions located in remote areas where contracting the services of a radiopharmacy is impracticable. 7

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Technetium-99 m generators 8

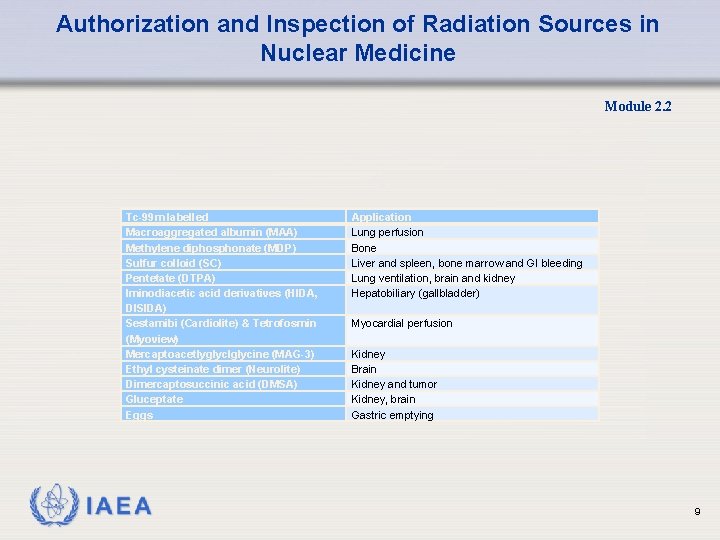
Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Tc-99 m labelled Macroaggregated albumin (MAA) Methylene diphosphonate (MDP) Sulfur colloid (SC) Pentetate (DTPA) Iminodiacetic acid derivatives (HIDA, DISIDA) Sestamibi (Cardiolite) & Tetrofosmin (Myoview) Mercaptoacetlyglyclglycine (MAG-3) Ethyl cysteinate dimer (Neurolite) Dimercaptosuccinic acid (DMSA) Gluceptate Eggs Application Lung perfusion Bone Liver and spleen, bone marrow and GI bleeding Lung ventilation, brain and kidney Hepatobiliary (gallbladder) Myocardial perfusion Kidney Brain Kidney and tumor Kidney, brain Gastric emptying 9

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Technetium 99 m. Tc labeled radiopharmaceuticals are easily produced by simply adding 99 m. Tc. O 4 to many choices of “cold kits”. 10

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Technetium 99 m (cont) In short, 99 m. Tc. O 4 is added to a vial containing a chemical compound that binds to the radionuclide. The result is a radiopharmaceutical which will be taken up in the designated organ for imaging (or analysis) with a gamma camera. 11

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Iodine 131 Iodine: • is produced in a reactor; • is used in diagnostic procedures involving the thyroid and also for the treatment of thyroid disorders; • can be administered in capsule or liquid solution form; • requires special precautions to be implemented during administration. 12

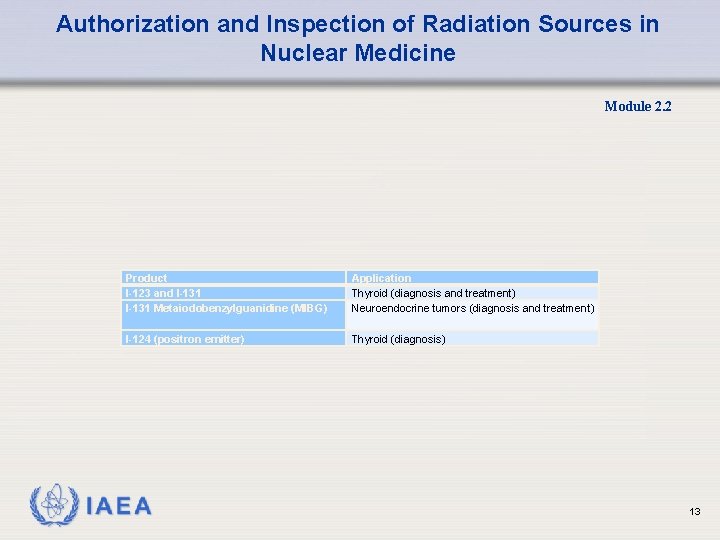
Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Product I-123 and I-131 Metaiodobenzylguanidine (MIBG) Application Thyroid (diagnosis and treatment) Neuroendocrine tumors (diagnosis and treatment) I-124 (positron emitter) Thyroid (diagnosis) 13

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Other Radionuclides The production of other radionuclides for nuclear medicine (e. g. PET) involves the use of a cyclotron. Medical Cyclotron Industrial cyclotron 14

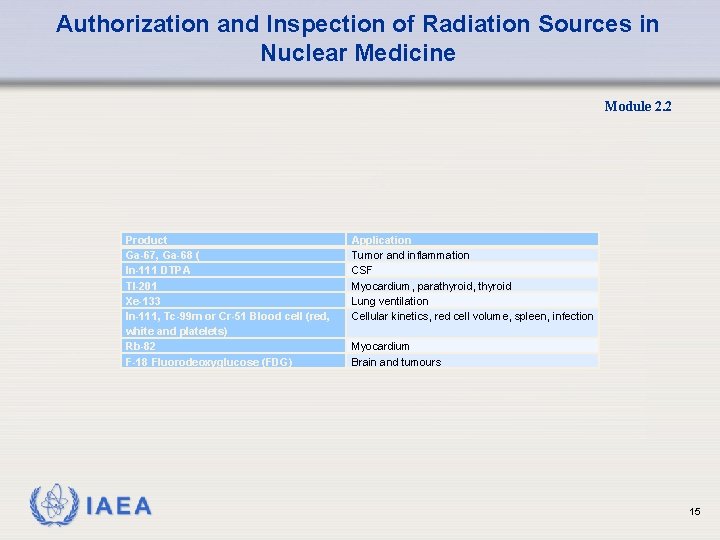
Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Product Ga-67, Ga-68 ( In-111 DTPA Tl-201 Xe-133 In-111, Tc-99 m or Cr-51 Blood cell (red, white and platelets) Rb-82 F-18 Fluorodeoxyglucose (FDG) Application Tumor and inflammation CSF Myocardium, parathyroid, thyroid Lung ventilation Cellular kinetics, red cell volume, spleen, infection Myocardium Brain and tumours 15

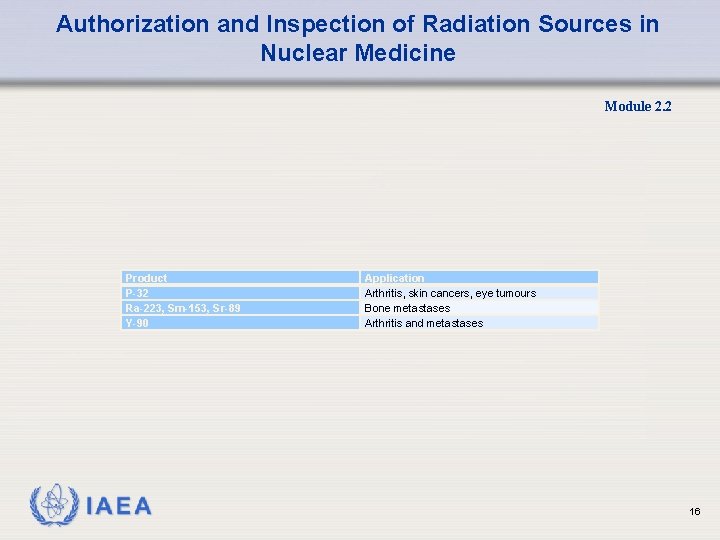
Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Product P-32 Ra-223, Sm-153, Sr-89 Y-90 Application Arthritis, skin cancers, eye tumours Bone metastases Arthritis and metastases 16

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Principles of Uptake (localization) A radiopharmaceutical: • has two components; a radionuclide and a pharmaceutical. In designing a radiopharmaceutical, a pharmaceutical is first chosen on the basis of its preferential uptake (localization) by a given organ or its participation in the physiological function of the organ i. e. the morphology and / or the physiology of the organ can be assessed. 17

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Good Manufacturing Practice • As radiopharmaceuticals are intended for human administration, it is essential that their manufacture is subject to strict quality control procedures. • These quality control procedures involve several specific tests and measurements that ensure the purity, potency, product identity, biologic safety, and efficacy of radiopharmaceuticals. 18

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Good Manufacturing Practice (cont) Radiopharmaceutical quality control tests fall into two categories: • physiochemical tests which indicate the level of radionuclide and radiochemical impurities and determine the p. H, ionic strength, osmolality and physical state of the sample, particularly if it is a colloid. • biologic tests which establish the sterility, non-pyrogenicity, and toxicity of the material. 19

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 Good Manufacturing Practice (cont) • Quality control tests for the “kits” used to prepare radiopharmaceuticals within a nuclear medicine department are performed at the manufacturer’s facility. • Generally, this is not an area of responsibility for the regulator and therefore is not reviewed by the inspector during a routine inspection. 20

Authorization and Inspection of Radiation Sources in Nuclear Medicine Module 2. 2 21
 What is a radiopharmaceutical
What is a radiopharmaceutical Labelling of radiopharmaceuticals
Labelling of radiopharmaceuticals Stability testing radiopharmaceuticals
Stability testing radiopharmaceuticals Print sources and web sources
Print sources and web sources Sújb
Sújb Ionizing radiation sources
Ionizing radiation sources Sources of radiation
Sources of radiation Importance of water sources
Importance of water sources Authentication filters in mvc 5
Authentication filters in mvc 5 Ohsu health services
Ohsu health services Authentication authorization accounting and auditing
Authentication authorization accounting and auditing Authentication and authorization infrastructure
Authentication and authorization infrastructure Rpd work authorization
Rpd work authorization Boee folder
Boee folder Postgraduate training authorization letter
Postgraduate training authorization letter Po box 31352 salt lake city ut 84131
Po box 31352 salt lake city ut 84131 Jmeter http authorization manager
Jmeter http authorization manager Autism authorization ctc
Autism authorization ctc Ac 90-105
Ac 90-105 Palawan pawnshop authorization letter
Palawan pawnshop authorization letter Eloccs registration guide
Eloccs registration guide Employment authorization document (ead)
Employment authorization document (ead)









































