Radiopharmaceuticals Definition of a Radiopharmaceutical A radiopharmaceutical is


















- Slides: 23

Radiopharmaceuticals

Definition of a Radiopharmaceutical A radiopharmaceutical is a radioactive compound used for the diagnosis and therapeutic treatment of human diseases. In nuclear medicine nearly 95% of the radiopharmaceuticals are used for diagnostic purposes, while the rest are used for therapeutic treatment. Radiopharmaceuticals usually have minimal pharmacologic effect, because in most cases they are used in tracer quantities. Therapeutic radiopharmaceuticals can cause tissue damage by radiation.

Definition of a Radiopharmaceutical Because they are administered to humans, they should be sterile and pyrogen free, and should undergo all quality control measures required of a conventional drug. A radiopharmaceutical may be a radioactive element such as 133 Xe, or a labeled compound such as 131 I-iodinated proteins and 99 m. Tc-labeled compounds.

Definition of a Radiopharmaceutical Although the term radiopharmaceutical is most • commonly used, other terms such as radiotracer, radiodiagnostic agent, and tracer have been used by various groups. We shall use the term radiopharmaceutical • throughout, although the term tracer will be used occasionally.

Definition of a Radiopharmaceutical • Another point of interest is the deference between radiochemicals and radiopharmaceuticals. • The former are not usable for administration to humans due to the possible lack of sterility and nonpyrogenicity. • On the other hand, radiopharmaceuticals are sterile and nonpyrogenic and can be administered safely to humans.

Definition of a Radiopharmaceutical A radiopharmaceutical has two components: a radionuclide and a pharmaceutical. The usefulness of a radiopharmaceutical is dictated by the characteristics of these two components. In designing a radiopharmaceutical, a pharmaceutical is first chosen on the basis of its preferential localization in a given organ or its participation in the physiologic function of the organ.

Definition of a Radiopharmaceutical Then a suitable radionuclide is tagged onto the chosen pharmaceutical such that after administration of the radiopharmaceutical, radiations emitted from it are detected by a radiation detector. Thus, the morphologic structure or the physiologic function of the organ can be assessed. The pharmaceutical of choice should be safe and nontoxic for human administration.

Definition of a Radiopharmaceutical • Radiations from the radionuclide of choice should be easily detected by nuclear instruments, and the radiation dose to the patient should be minimal.

IDEAL RADIOPHARMACEUTICAL

Ideal Radiopharmaceutical • Since radiopharmaceuticals are administered to humans, and because there are several limitations on the detection of radiations by currently available instruments, radiopharmaceuticals should possess some important characteristics. • The ideal characteristics for radiopharmaceuticals are:

Ideal radiopharmaceutical Easy availability Short effective Half-Life Minimal Particle Emission Decay by Electron Capture or Isomeric Transition • High Target-to Non target Activity Ratio • •

Ide 1. Easy Availability al Rad The radiopharmaceutical should be easily produced, inexpensive, and readily available in any nuclear iop medicine facility har Complicated methods of production of radionuclides or ma labeled compounds increase the cost of the radiopharmaceutical. ceu tica The geographic distance between the user and the supplier also limits the availability of short-lived l radiopharmaceuticals.

Ide al Rad iop har ma ceu tica l 2. Short Effective Half-Life A radionuclide decays with a definite half-life, which is called the physical half -life, denoted Tp (or t 1/2). The physical half-life is independent of any physicochemical condition and is characteristic for a given radionuclide

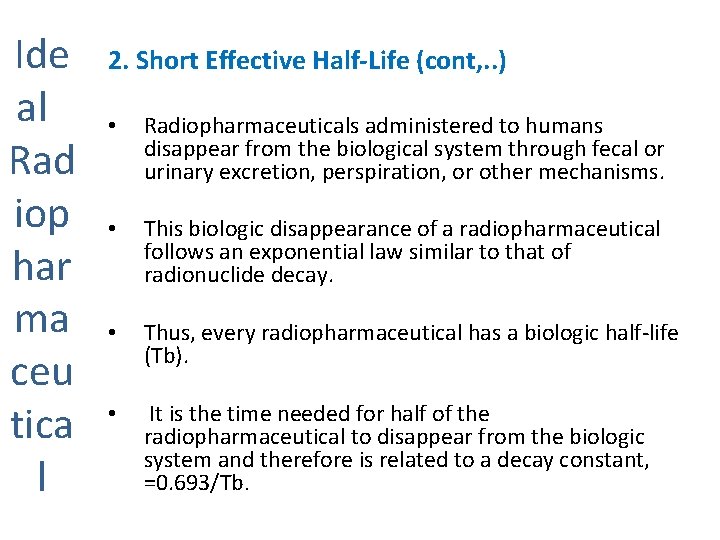
Ide al Rad iop har ma ceu tica l 2. Short Effective Half-Life (cont, . . ) • Radiopharmaceuticals administered to humans disappear from the biological system through fecal or urinary excretion, perspiration, or other mechanisms. • This biologic disappearance of a radiopharmaceutical follows an exponential law similar to that of radionuclide decay. • Thus, every radiopharmaceutical has a biologic half-life (Tb). • It is the time needed for half of the radiopharmaceutical to disappear from the biologic system and therefore is related to a decay constant, =0. 693/Tb.

Ide al Rad iop har ma ceu tica l 2. Short Effective Half-Life (cont, . . ) Obviously, in any biologic system, the loss of a radiopharmaceutical is due to both the physical decay of the radionuclide and the biologic elimination of the radiopharmaceutical.

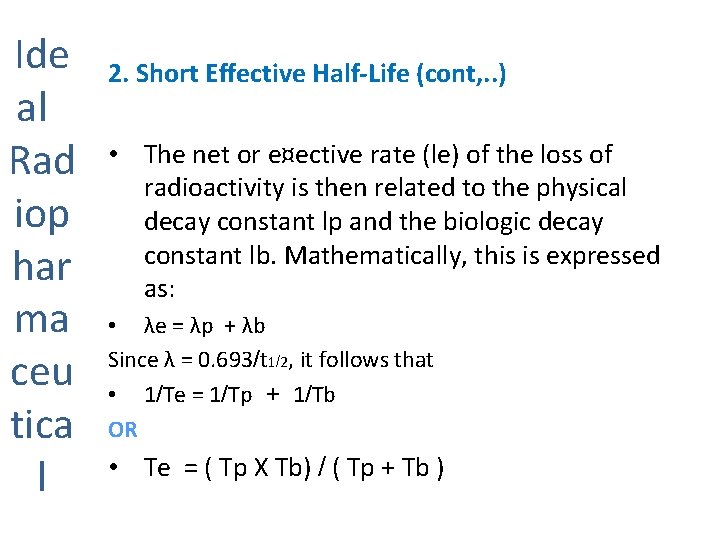
Ide al Rad iop har ma ceu tica l 2. Short Effective Half-Life (cont, . . ) • The net or e¤ective rate (le) of the loss of radioactivity is then related to the physical decay constant lp and the biologic decay constant lb. Mathematically, this is expressed as: • λe = λp + λb Since λ = 0. 693/t 1/2, it follows that • 1/Te = 1/Tp + 1/Tb OR • Te = ( Tp X Tb) / ( Tp + Tb )

Problem : The physical half-life of 111 In is 67 hr and the biologic half-life of 111 In-DTPA used for measurement of the glomerular filtration rate is 1. 5 hr. What is the effective half-life of 111 In. DTPA? Answer Using Eq. Te = ( Tp X Tb) / ( Tp + Tb ) Te = 1. 46 h

3 - Particle Emission

Ide al Rad iop har ma ceu tica l 4. Decay by Electron Capture or Isomeric Transition Because radionuclides emitting particles are less desirable, the diagnostic radionuclides used should decay by electron capture or isomeric transition without any internal conversion. Whatever the mode of decay, for diagnostic studies the radionuclide must emit a Ɣ radiation with an energy preferably between 30 and 300 ke. V. Below 30 ke. V, Ɣ rays are absorbed by tissue

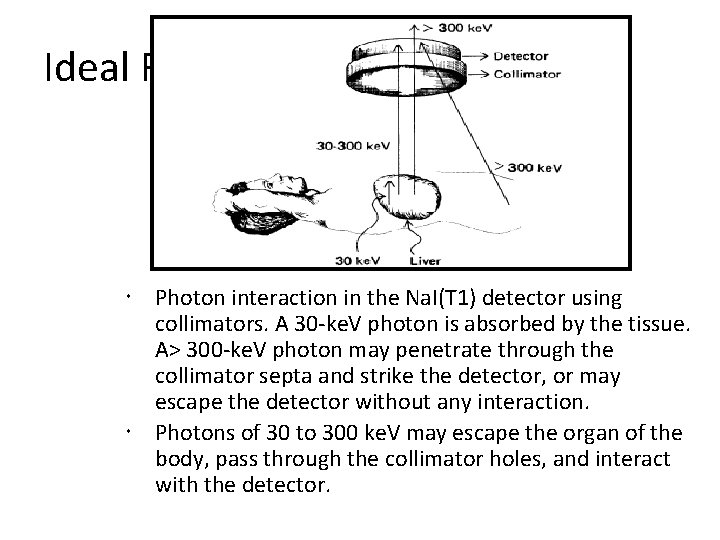
Ideal Radiopharmaceutical Photon interaction in the Na. I(T 1) detector using collimators. A 30 -ke. V photon is absorbed by the tissue. A> 300 -ke. V photon may penetrate through the collimator septa and strike the detector, or may escape the detector without any interaction. Photons of 30 to 300 ke. V may escape the organ of the body, pass through the collimator holes, and interact with the detector.

5 -High Target-to-Non target Activity Ratio: For any diagnostic study, it is desirable that the radiopharmaceutical be localized preferentially in the organ under study since the activity from nontarget areas can obscure the structural details of the picture of the target organ. Therefore, the target-to-non target activity ratio should be large. An ideal radiopharmaceutical should have all the above characteristics to provide maximum efficacy in the diagnosis of diseases and a minimum radiation

Radiopharmaceuticals • Radioactive element - 133 Xe • Labeled compounds - 131 I iodinated proteins • 99 m. Tc labeled compounds • [18 F]FDG

Production of Radionuclides • Reactor-Produced Radionuclides • Cyclotron-Produced Radionuclides • Generators
 Radiopharmaceuticals definition
Radiopharmaceuticals definition Dmsa test
Dmsa test Nevada radiation control program
Nevada radiation control program Radiolabeling definition
Radiolabeling definition Stability testing radiopharmaceuticals
Stability testing radiopharmaceuticals Definition essay examples
Definition essay examples Operational definition examples
Operational definition examples Soziale wirklichkeit definition
Soziale wirklichkeit definition Energiereserven
Energiereserven Meaning
Meaning What does a career portfolio look like
What does a career portfolio look like Bildungsroman meaning
Bildungsroman meaning Tanzimat reforms definition
Tanzimat reforms definition Coconut shy definition
Coconut shy definition Define internal conflict
Define internal conflict Jonathan edwards apush definition
Jonathan edwards apush definition Marbury vs madison apush
Marbury vs madison apush Mini saga stories
Mini saga stories Narrative essay meaning
Narrative essay meaning Apush leq rubric
Apush leq rubric What is expository paragraph?
What is expository paragraph? Sensory
Sensory Objective summary verbs
Objective summary verbs Objectives of summary writing
Objectives of summary writing