A NEW LOOK AT RA Interactive Hot Topics






- Slides: 27

A NEW LOOK AT RA Interactive Hot Topics Series Understanding the Pharmacokinetics of Methotrexate: It’s Not So Simple MP-RA-0285

Pharmacokinetics: What Does the Body Do to the Drug? § Absorption from the subcutaneous site into the circulation § Distribution to tissue compartments or binding to plasma proteins (albumin) § Metabolism and degradation after absorption into circulation § Excretion via both renal and hepatic mechanisms Pharmacodynamics: What Does the Drug Do to the Body? 2

Absorption

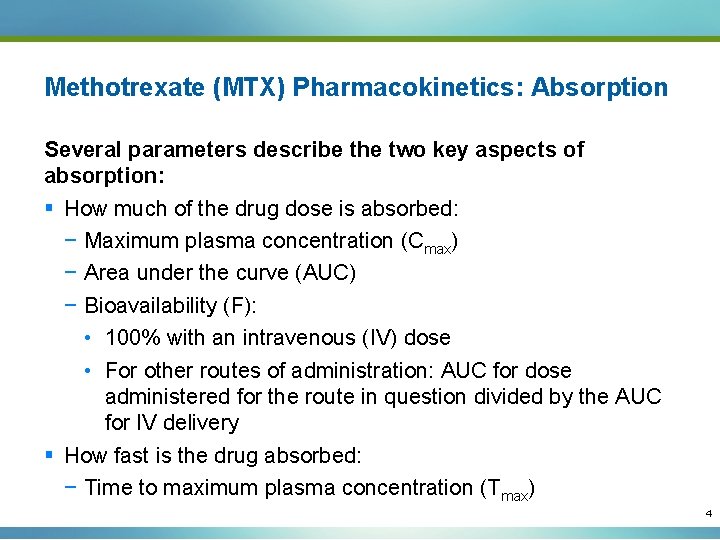
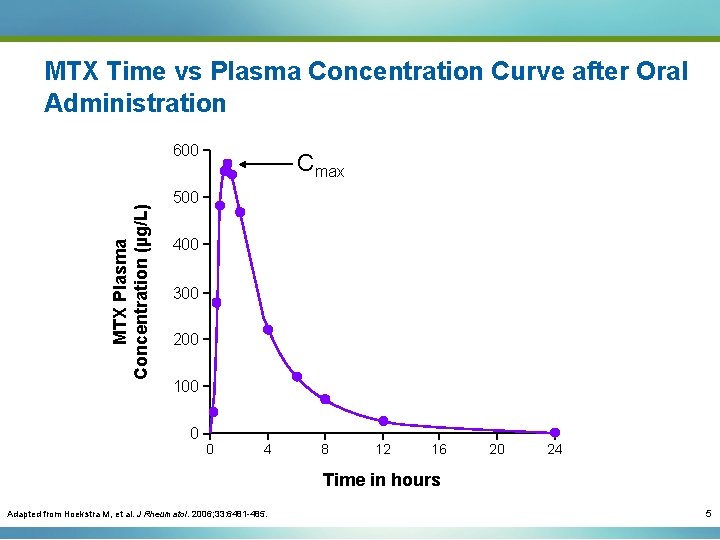
Methotrexate (MTX) Pharmacokinetics: Absorption Several parameters describe the two key aspects of absorption: § How much of the drug dose is absorbed: − Maximum plasma concentration (Cmax) − Area under the curve (AUC) − Bioavailability (F): • 100% with an intravenous (IV) dose • For other routes of administration: AUC for dose administered for the route in question divided by the AUC for IV delivery § How fast is the drug absorbed: − Time to maximum plasma concentration (Tmax) 4

MTX Time vs Plasma Concentration Curve after Oral Administration MTX Plasma Concentration (µg/L) 600 Cmax 500 400 300 200 100 0 0 4 8 12 16 20 24 Time in hours Adapted from Hoekstra M, et al. J Rheumatol. 2006; 33: 6481 -485. 5

MTX Time vs Plasma Concentration Curve after Oral Administration MTX Plasma Concentration (µg/L) 600 500 400 AUC 300 200 100 0 0 4 8 12 16 20 24 Time in hours Adapted from Hoekstra M, et al. J Rheumatol. 2006; 33: 6481 -485. 6

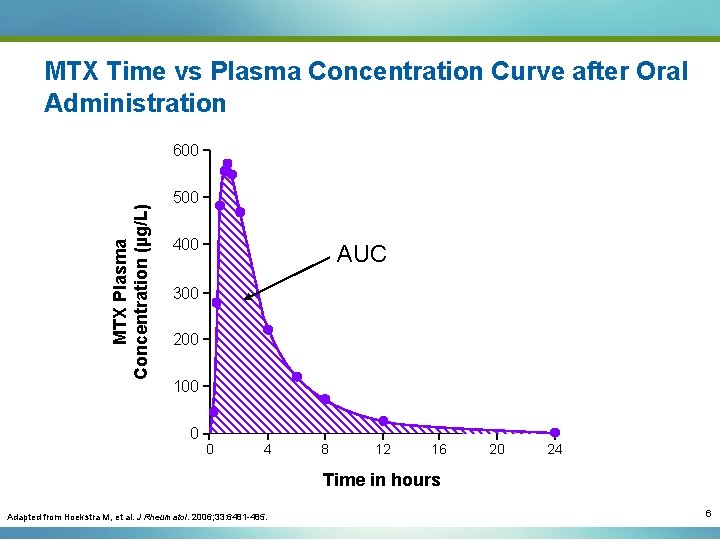
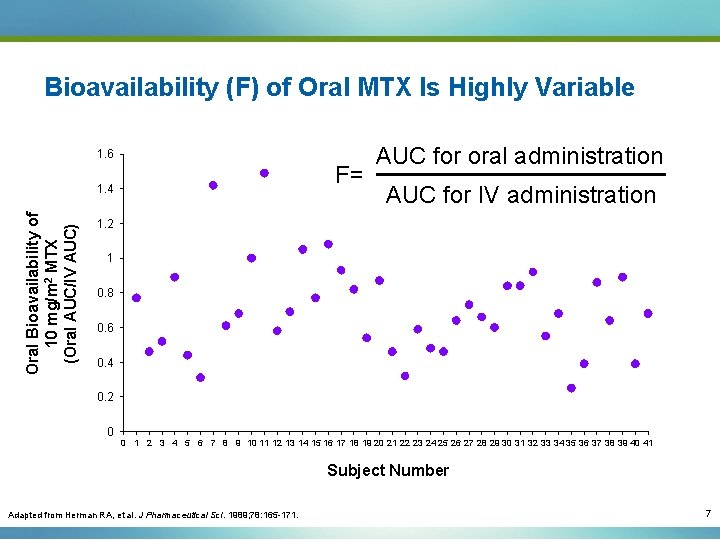
Bioavailability (F) of Oral MTX Is Highly Variable 1. 6 F= Oral Bioavailability of 10 mg/m 2 MTX (Oral AUC/IV AUC) 1. 4 AUC for oral administration AUC for IV administration 1. 2 1 0. 8 0. 6 0. 4 0. 2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 Subject Number Adapted from Herman RA, et al. J Pharmaceutical Sci. 1989; 78: 165 -171. 7

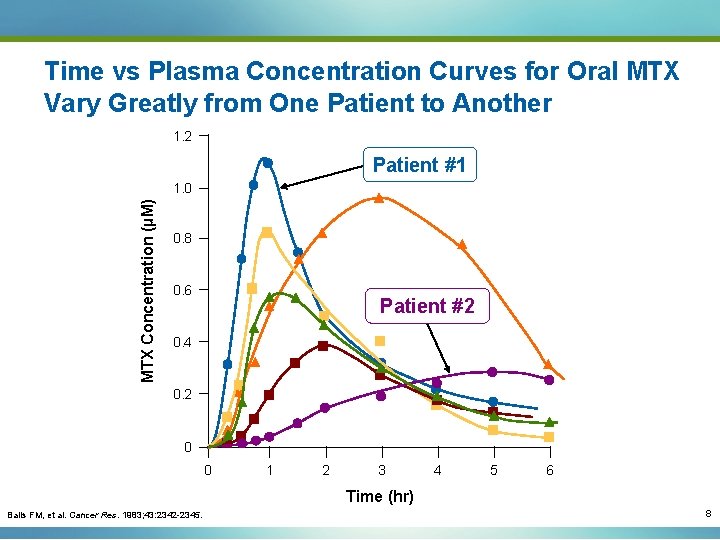
Time vs Plasma Concentration Curves for Oral MTX Vary Greatly from One Patient to Another 1. 2 Patient #1 MTX Concentration (µM) 1. 0 0. 8 0. 6 Patient #2 0. 4 0. 2 0 0 1 2 3 4 5 6 Time (hr) Balis FM, et al. Cancer Res. 1983; 43: 2342 -2345. 8

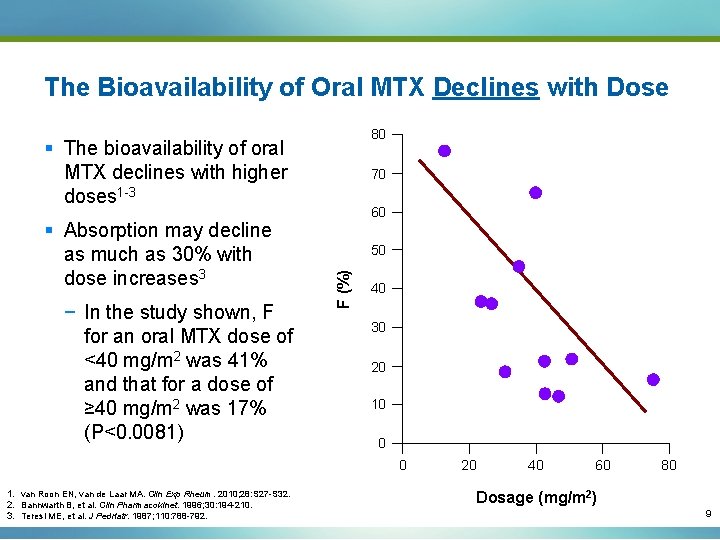
The Bioavailability of Oral MTX Declines with Dose 80 § The bioavailability of oral MTX declines with higher doses 1 -3 − In the study shown, F for an oral MTX dose of <40 mg/m 2 was 41% and that for a dose of ≥ 40 mg/m 2 was 17% (P<0. 0081) 60 50 F (%) § Absorption may decline as much as 30% with dose increases 3 70 40 30 20 10 0 0 1. van Roon EN, van de Laar MA. Clin Exp Rheum. 2010; 28: S 27 -S 32. 2. Bannwarth B, et al. Clin Pharmacokinet. 1996; 30: 194 -210. 3. Teresi ME, et al. J Pedriatr. 1987; 110: 788 -792. 20 40 60 80 Dosage (mg/m 2) 9

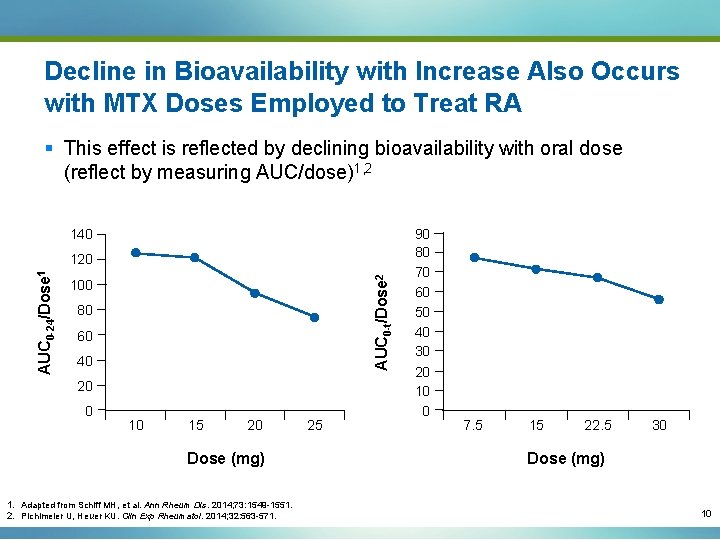
Decline in Bioavailability with Increase Also Occurs with MTX Doses Employed to Treat RA § This effect is reflected by declining bioavailability with oral dose (reflect by measuring AUC/dose)1, 2 140 AUC 0 -t/Dose 2 AUC 0 -24/Dose 1 120 100 80 60 40 20 0 10 15 20 Dose (mg) 1. Adapted from Schiff MH, et al. Ann Rheum Dis. 2014; 73: 1549 -1551. 2. Pichlmeier U, Heuer KU. Clin Exp Rheumatol. 2014; 32: 563 -571. 25 90 80 70 60 50 40 30 20 10 0 7. 5 15 22. 5 30 Dose (mg) 10

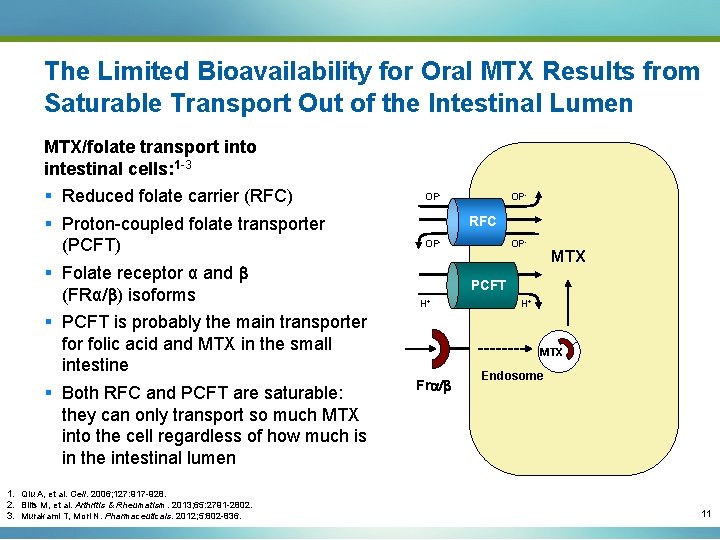
The Limited Bioavailability for Oral MTX Results from Saturable Transport Out of the Intestinal Lumen MTX/folate transport into intestinal cells: 1 -3 § Reduced folate carrier (RFC) § Proton-coupled folate transporter (PCFT) § Folate receptor α and b (FRα/b) isoforms § PCFT is probably the main transporter folic acid and MTX in the small intestine § Both RFC and PCFT are saturable: they can only transport so much MTX into the cell regardless of how much is in the intestinal lumen 1. Qiu A, et al. Cell. 2006; 127: 917 -928. 2. Blits M, et al. Arthritis & Rheumatism. 2013; 65: 2791 -2802. 3. Murakami T, Mori N. Pharmaceuticals. 2012; 5: 802 -836. OP- RFC OP- MTX PCFT H+ H+ MTX Fra/b Endosome 11

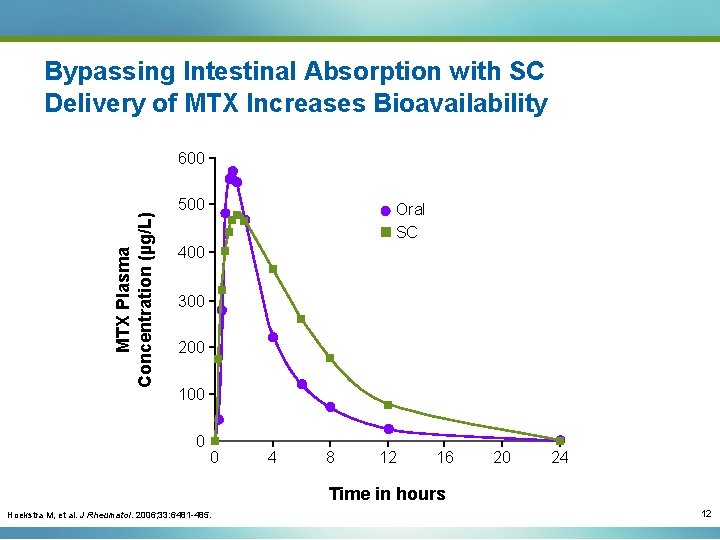
Bypassing Intestinal Absorption with SC Delivery of MTX Increases Bioavailability MTX Plasma Concentration (µg/L) 600 500 Oral SC 400 300 200 100 0 0 4 8 12 16 20 24 Time in hours Hoekstra M, et al. J Rheumatol. 2006; 33: 6481 -485. 12

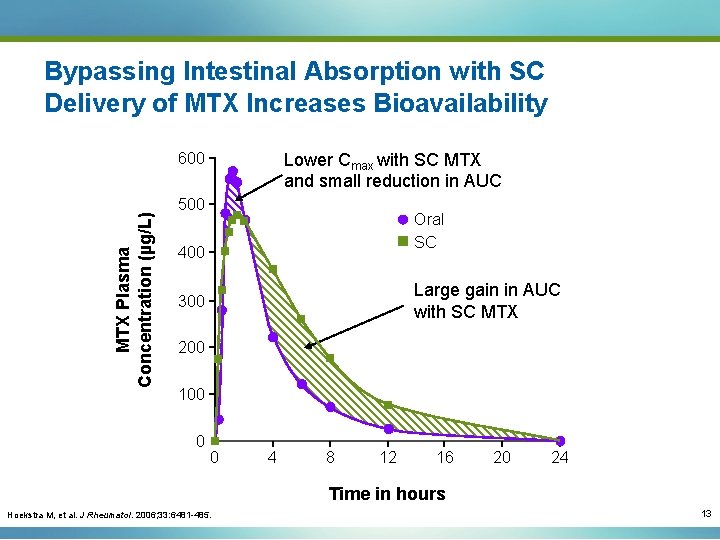
Bypassing Intestinal Absorption with SC Delivery of MTX Increases Bioavailability MTX Plasma Concentration (µg/L) 600 Lower Cmax with SC MTX and small reduction in AUC 500 Oral SC 400 Large gain in AUC with SC MTX 300 200 100 0 0 4 8 12 16 20 24 Time in hours Hoekstra M, et al. J Rheumatol. 2006; 33: 6481 -485. 13

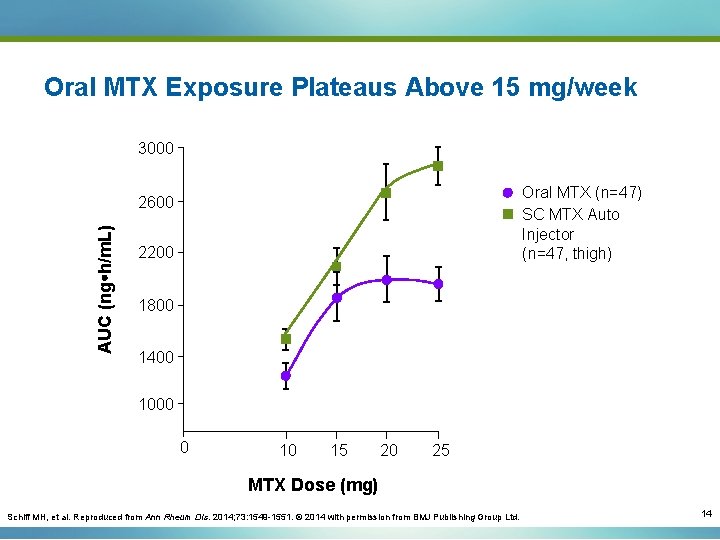
Oral MTX Exposure Plateaus Above 15 mg/week 3000 Oral MTX (n=47) SC MTX Auto Injector (n=47, thigh) AUC (ng h/m. L) 2600 2200 1800 1400 1000 0 10 15 20 25 MTX Dose (mg) Schiff MH, et al. Reproduced from Ann Rheum Dis. 2014; 73: 1549 -1551. © 2014 with permission from BMJ Publishing Group Ltd. 14

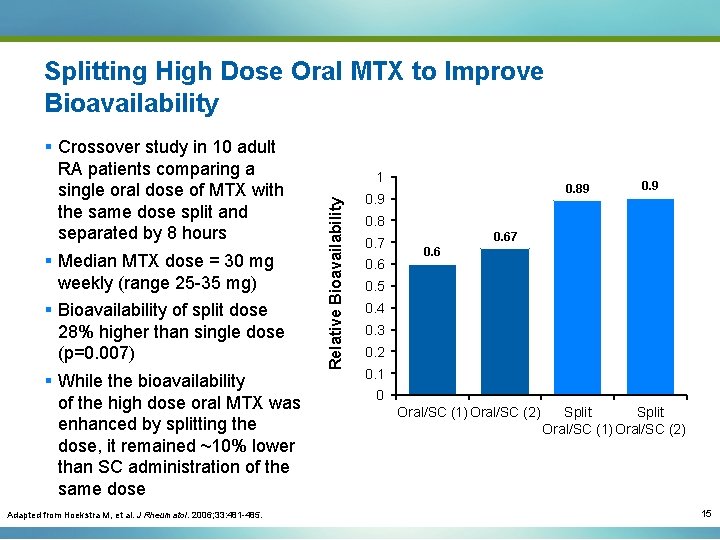
Splitting High Dose Oral MTX to Improve Bioavailability Adapted from Hoekstra M, et al. J Rheumatol. 2006; 33: 481 -485. 1 Relative Bioavailability § Crossover study in 10 adult RA patients comparing a single oral dose of MTX with the same dose split and separated by 8 hours § Median MTX dose = 30 mg weekly (range 25 -35 mg) § Bioavailability of split dose 28% higher than single dose (p=0. 007) § While the bioavailability of the high dose oral MTX was enhanced by splitting the dose, it remained ~10% lower than SC administration of the same dose 0. 89 0. 8 0. 7 0. 67 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 Oral/SC (1) Oral/SC (2) Split Oral/SC (1) Oral/SC (2) 15

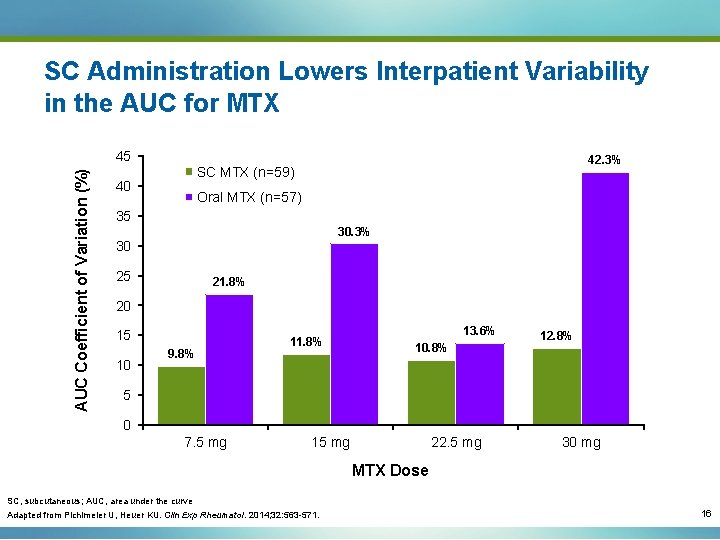
SC Administration Lowers Interpatient Variability in the AUC for MTX AUC Coefficient of Variation (%) 45 42. 3% SC MTX (n=59) 40 Oral MTX (n=57) 35 30. 3% 30 25 21. 8% 20 15 10 9. 8% 11. 8% 13. 6% 10. 8% 12. 8% 5 0 7. 5 mg 15 mg 22. 5 mg 30 mg MTX Dose SC, subcutaneous; AUC, area under the curve Adapted from Pichlmeier U, Heuer KU. Clin Exp Rheumatol. 2014; 32: 563 -571. 16

Distribution

Metabolism

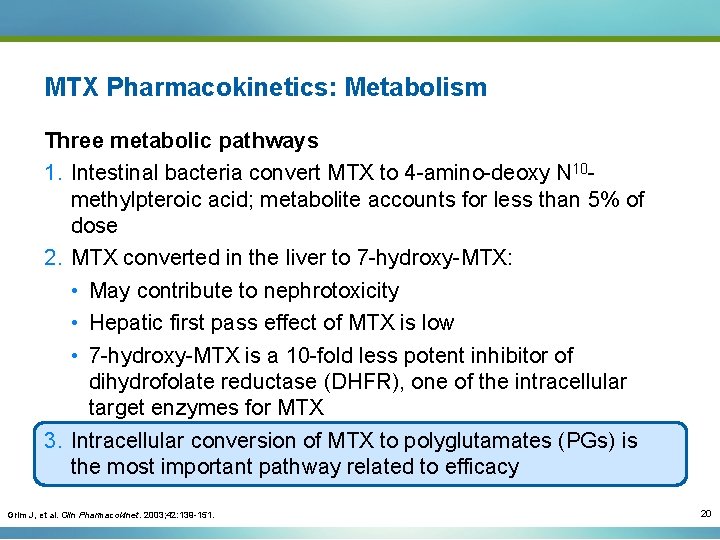
MTX Pharmacokinetics: Metabolism Three metabolic pathways 1. Intestinal bacteria convert MTX to 4 -amino-deoxy N 10 methylpteroic acid; metabolite accounts for less than 5% of dose 2. MTX converted in the liver to 7 -hydroxy-MTX: • May contribute to nephrotoxicity • Hepatic first pass effect of MTX is low • 7 -hydroxy-MTX is a 10 -fold less potent inhibitor of dihydrofolate reductase (DHFR), one of the intracellular target enzymes for MTX 3. Intracellular conversion of MTX to polyglutamates (PGs) is the most important pathway related to efficacy Grim J, et al. Clin Pharmacokinet. 2003; 42: 139 -151. 20

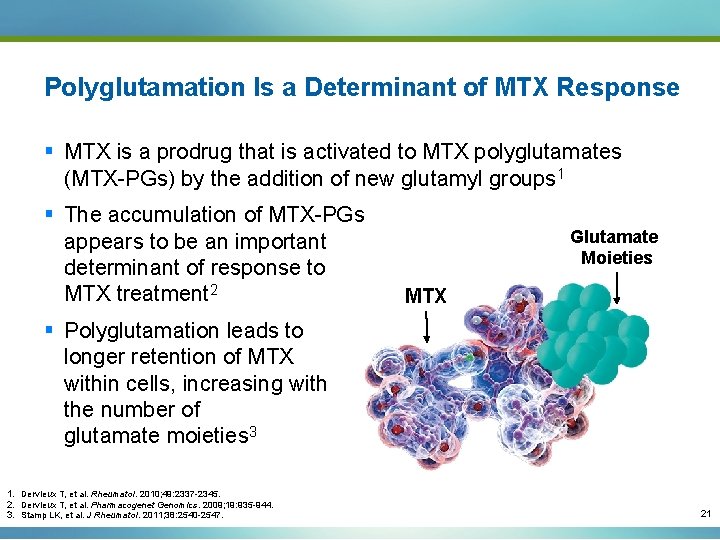
Polyglutamation Is a Determinant of MTX Response § MTX is a prodrug that is activated to MTX polyglutamates (MTX-PGs) by the addition of new glutamyl groups 1 § The accumulation of MTX-PGs appears to be an important determinant of response to MTX treatment 2 Glutamate Moieties MTX § Polyglutamation leads to longer retention of MTX within cells, increasing with the number of glutamate moieties 3 1. Dervieux T, et al. Rheumatol. 2010; 49: 2337 -2345. 2. Dervieux T, et al. Pharmacogenet Genomics. 2009; 19: 935 -944. 3. Stamp LK, et al. J Rheumatol. 2011; 38: 2540 -2547. 21

Pharmacodynamics

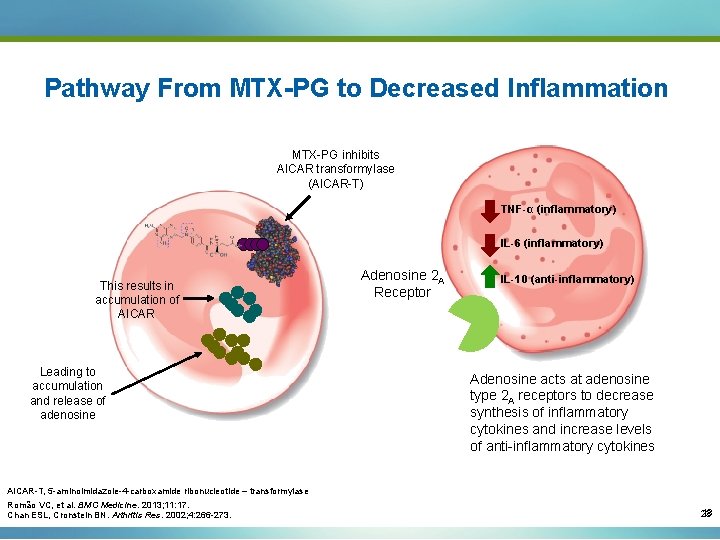
Pathway From MTX-PG to Decreased Inflammation MTX-PG inhibits AICAR transformylase (AICAR-T) TNF-α (inflammatory) IL-6 (inflammatory) This results in accumulation of AICAR Leading to accumulation and release of adenosine Adenosine 2 A Receptor IL-10 (anti-inflammatory) Adenosine acts at adenosine type 2 A receptors to decrease synthesis of inflammatory cytokines and increase levels of anti-inflammatory cytokines AICAR-T, 5 -aminoimidazole-4 -carboxamide ribonucleotide – transformylase Romão VC, et al. BMC Medicine. 2013; 11: 17. Chan ESL, Cronstein BN. Arthritis Res. 2002; 4: 266 -273. 18 23

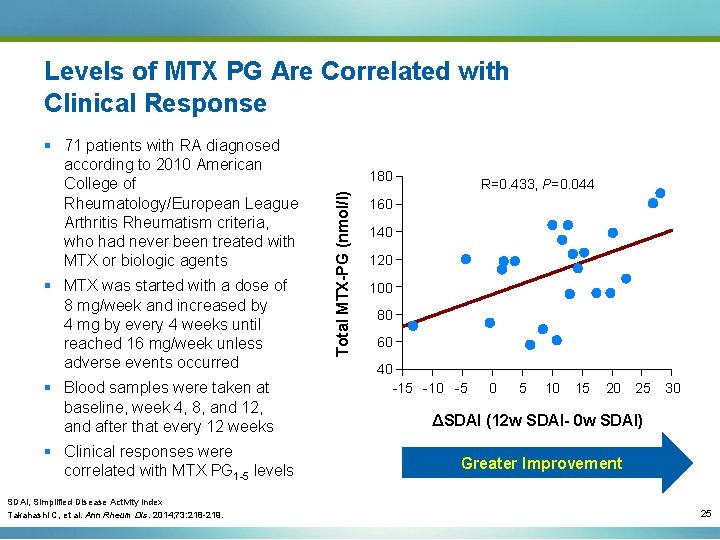
Levels of MTX PG Are Correlated with Clinical Response § MTX was started with a dose of 8 mg/week and increased by 4 mg by every 4 weeks until reached 16 mg/week unless adverse events occurred § Blood samples were taken at baseline, week 4, 8, and 12, and after that every 12 weeks § Clinical responses were correlated with MTX PG 1 -5 levels 180 Total MTX-PG (nmol/l) § 71 patients with RA diagnosed according to 2010 American College of Rheumatology/European League Arthritis Rheumatism criteria, who had never been treated with MTX or biologic agents R=0. 433, P=0. 044 160 140 120 100 80 60 40 -15 -10 -5 0 5 10 15 20 25 30 ΔSDAI (12 w SDAI- 0 w SDAI) Greater Improvement SDAI, Simplified Disease Activity Index Takahashi C, et al. Ann Rheum Dis. 2014; 73: 218 -219. 25

Elimination

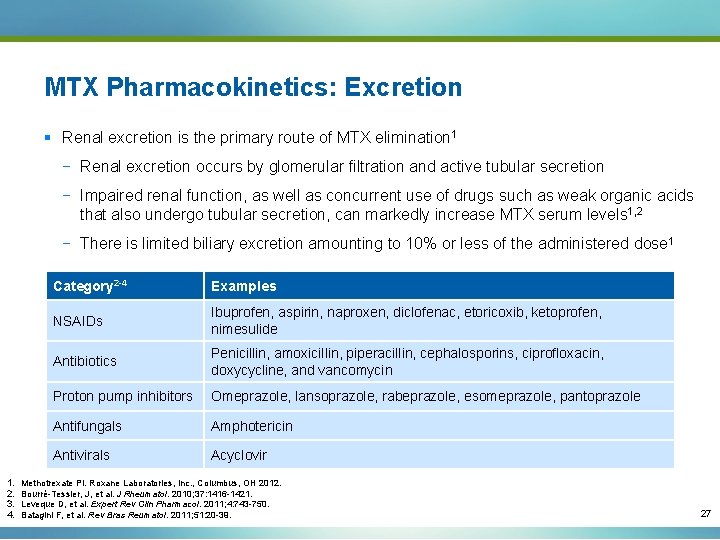
MTX Pharmacokinetics: Excretion § Renal excretion is the primary route of MTX elimination 1 − Renal excretion occurs by glomerular filtration and active tubular secretion − Impaired renal function, as well as concurrent use of drugs such as weak organic acids that also undergo tubular secretion, can markedly increase MTX serum levels 1, 2 − There is limited biliary excretion amounting to 10% or less of the administered dose 1 1. 2. 3. 4. Category 2 -4 Examples NSAIDs Ibuprofen, aspirin, naproxen, diclofenac, etoricoxib, ketoprofen, nimesulide Antibiotics Penicillin, amoxicillin, piperacillin, cephalosporins, ciprofloxacin, doxycycline, and vancomycin Proton pump inhibitors Omeprazole, lansoprazole, rabeprazole, esomeprazole, pantoprazole Antifungals Amphotericin Antivirals Acyclovir Methotrexate PI. Roxane Laboratories, Inc. , Columbus, OH 2012. Bourré-Tessier, J, et al. J Rheumatol. 2010; 37: 1416 -1421. Leveque D, et al. Expert Rev Clin Pharmacol. 2011; 4: 743 -750. Batagini F, et al. Rev Bras Reumatol. 2011; 51: 20 -39. 27

Summary

Summary: MTX Pharmacokinetics (cont. ) § Oral route of administration represents a limiting factor when attempting to optimize MTX dosing 1 § Given that absorption varies greatly between oral and parenteral routes, switching from oral to parenteral may be an important option to improve patient outcomes 2 1. van Roon EN, van de Laar MA. Clin Exp Rheum. 2010; 28: S 27 -S 32. 2. Braun J, et al. Arthritis Rheum. 2008; 58: 73 -81. 29