7 7 Fischer Projections Purpose of Fischer projections














![CO 2 H HO H [a] = -9. 5° CO 2 H [a] = CO 2 H HO H [a] = -9. 5° CO 2 H [a] =](https://slidetodoc.com/presentation_image_h2/bd07b7a33d2b594bfa1e589bf3782ccd/image-27.jpg)
![CO 2 H HO H [a] = -9. 5° CO 2 H [a] = CO 2 H HO H [a] = -9. 5° CO 2 H [a] =](https://slidetodoc.com/presentation_image_h2/bd07b7a33d2b594bfa1e589bf3782ccd/image-30.jpg)






- Slides: 51

7. 7 Fischer Projections • Purpose of Fischer projections is to show configuration at chirality center without necessity of drawing wedges and dashes or using models. Dr. Wolf's CHM 201 & 202 1

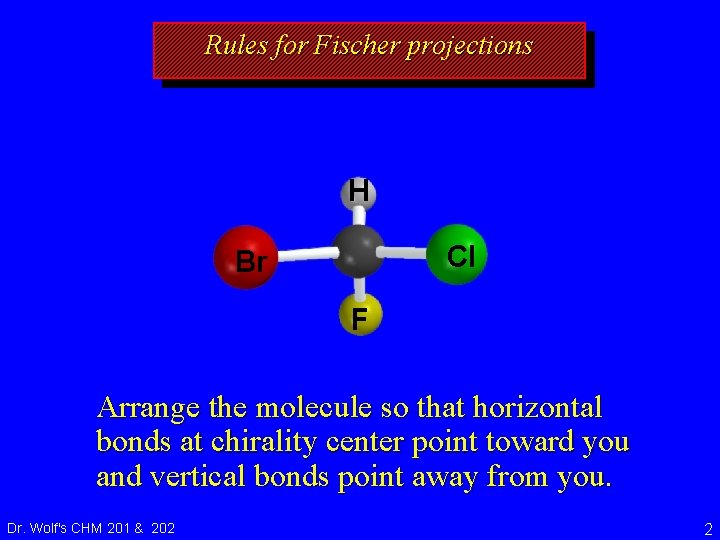
Rules for Fischer projections H Cl Br F Arrange the molecule so that horizontal bonds at chirality center point toward you and vertical bonds point away from you. Dr. Wolf's CHM 201 & 202 2

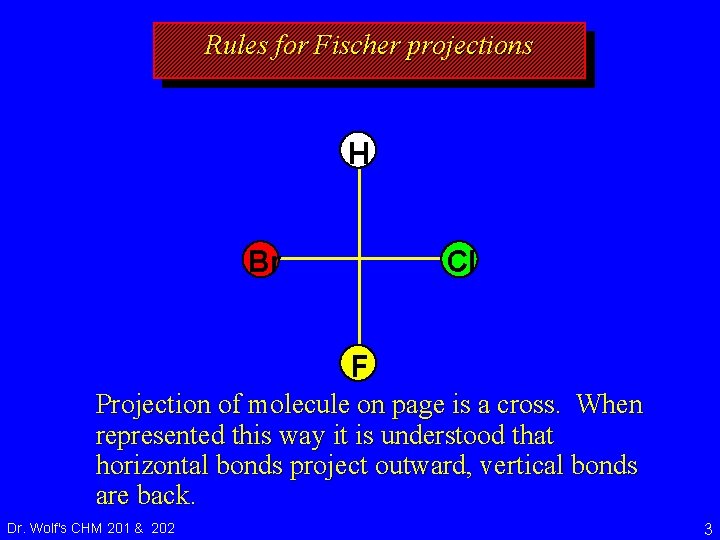
Rules for Fischer projections H Br Cl F Projection of molecule on page is a cross. When represented this way it is understood that horizontal bonds project outward, vertical bonds are back. Dr. Wolf's CHM 201 & 202 3

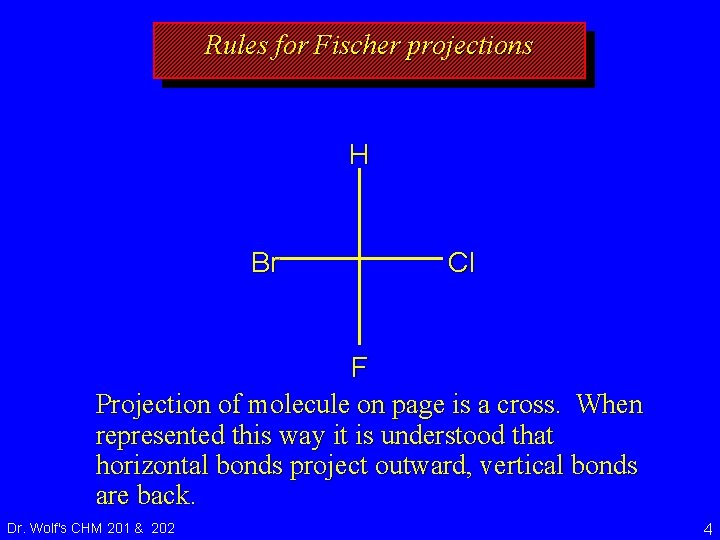
Rules for Fischer projections H Br Cl F Projection of molecule on page is a cross. When represented this way it is understood that horizontal bonds project outward, vertical bonds are back. Dr. Wolf's CHM 201 & 202 4

7. 8 Physical Properties of Enantiomers Dr. Wolf's CHM 201 & 202 5

Physical properties of enantiomers Same: melting point, boiling point, density, etc Different: properties that depend on shape of molecule (biological-physiological properties) can be different Dr. Wolf's CHM 201 & 202 6

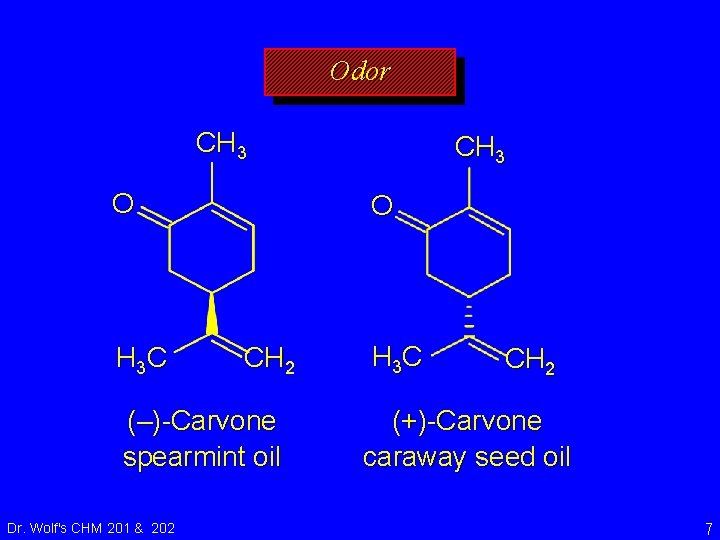
Odor CH 3 O H 3 C O CH 2 (–)-Carvone spearmint oil Dr. Wolf's CHM 201 & 202 CH 3 H 3 C CH 2 (+)-Carvone caraway seed oil 7

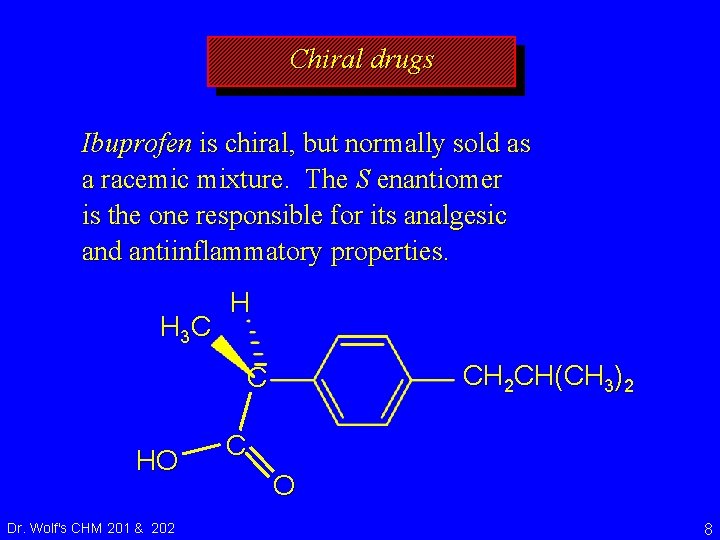
Chiral drugs Ibuprofen is chiral, but normally sold as a racemic mixture. The S enantiomer is the one responsible for its analgesic and antiinflammatory properties. H 3 C H CH 2 CH(CH 3)2 C HO Dr. Wolf's CHM 201 & 202 C O 8

7. 9 Reactions That Create A Chiral Center Dr. Wolf's CHM 201 & 202 9

Many reactions convert achiral reactants to chiral products. It is important to recognize, however, that if all of the components of the starting state (reactants, catalysts, solvents, etc. ) are achiral, any chiral product will be formed as a racemic mixture. This generalization can be more simply stated as "Optically inactive starting materials can't give optically active products. " (Remember: In order for a substance to be optically active, it must be chiral and one enantiomer must be present in greater amounts than the other. Dr. Wolf's CHM 201 & 202 10

Example O CH 3 CH CH 2 H CH 3 COOH H 3 C C CH 2 O Achiral Dr. Wolf's CHM 201 & 202 Chiral, but racemic 11

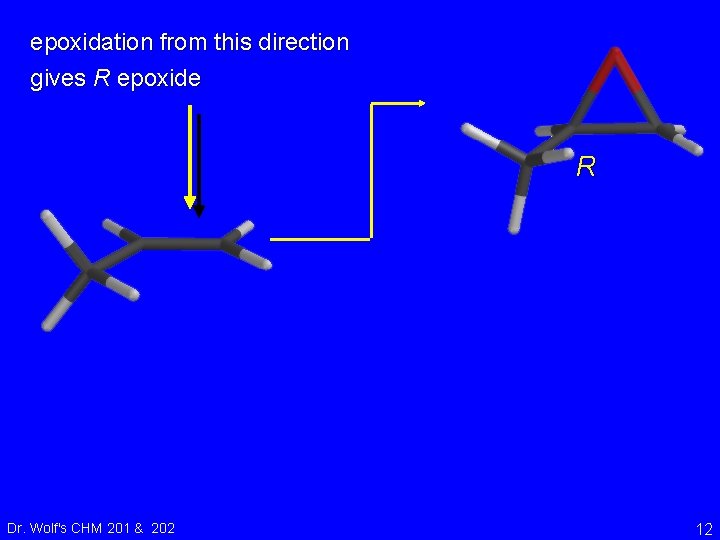
epoxidation from this direction gives R epoxide R Dr. Wolf's CHM 201 & 202 12

epoxidation from this direction gives R epoxide R S epoxidation from this direction gives S epoxide Dr. Wolf's CHM 201 & 202 13

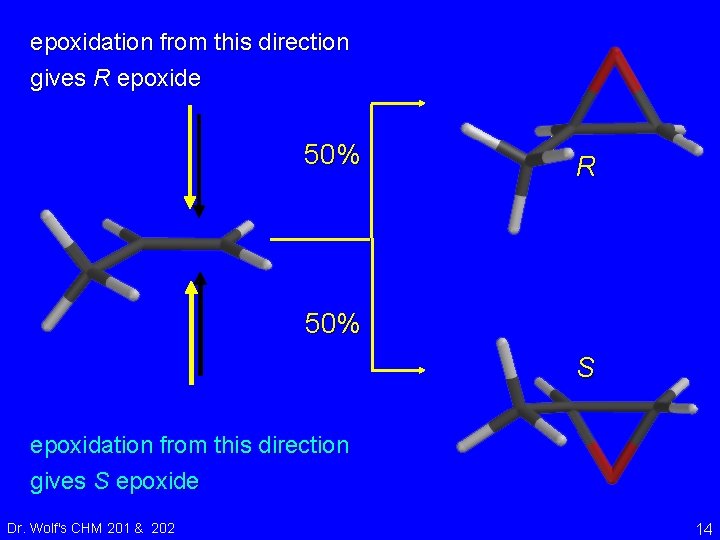
epoxidation from this direction gives R epoxide 50% R 50% S epoxidation from this direction gives S epoxide Dr. Wolf's CHM 201 & 202 14

Example Br 2, H 2 O CH 3 CH CH 2 CH 3 CHCH 2 Br OH Achiral Dr. Wolf's CHM 201 & 202 Chiral, but racemic 15

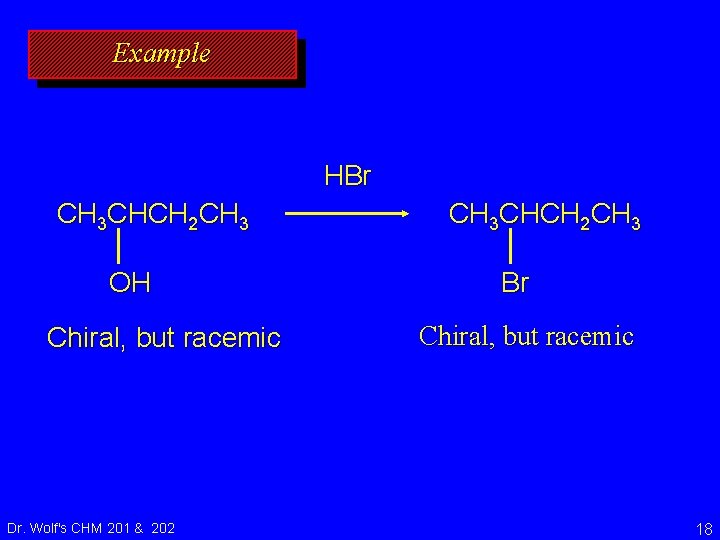
Example HBr CH 3 CH CHCH 3 CHCH 2 CH 3 Br Achiral Dr. Wolf's CHM 201 & 202 Chiral, but racemic 16

Many reactions convert chiral reactants to chiral products. However, if the reactant is racemic, the product will be racemic also. Remember: "Optically inactive starting materials can't give optically active products. " Dr. Wolf's CHM 201 & 202 17

Example HBr CH 3 CHCH 2 CH 3 OH Chiral, but racemic Dr. Wolf's CHM 201 & 202 CH 3 CHCH 2 CH 3 Br Chiral, but racemic 18

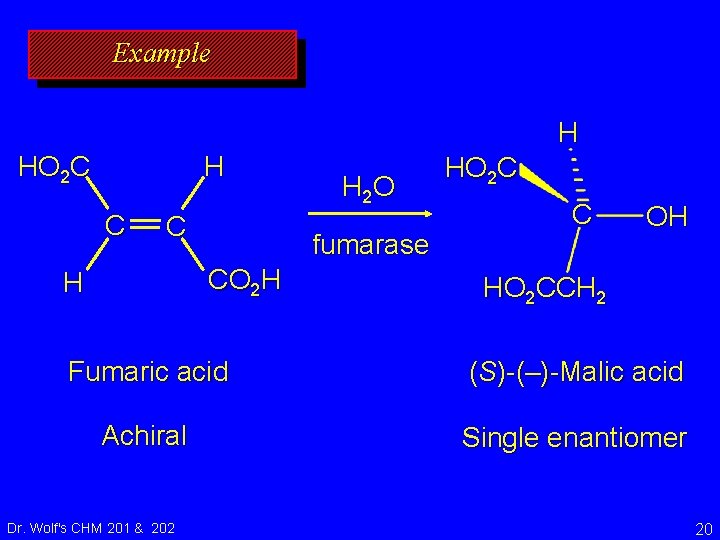
Many biochemical reactions convert an achiral reactant to a single enantiomer of a chiral product Reactions in living systems are catalyzed by enzymes, which are enantiomerically homogeneous. The enzyme (catalyst) is part of the reacting system, so such reactions don't violate the generalization that "Optically inactive starting materials can't give optically active products. " Dr. Wolf's CHM 201 & 202 19

Example H HO 2 C H C C fumarase CO 2 H H H 2 O HO 2 C C OH HO 2 CCH 2 Fumaric acid (S)-(–)-Malic acid Achiral Single enantiomer Dr. Wolf's CHM 201 & 202 20

7. 10 Chiral Molecules with Two Chirality Centers How many stereoisomers when a particular molecule contains two chiral centers? Dr. Wolf's CHM 201 & 202 21

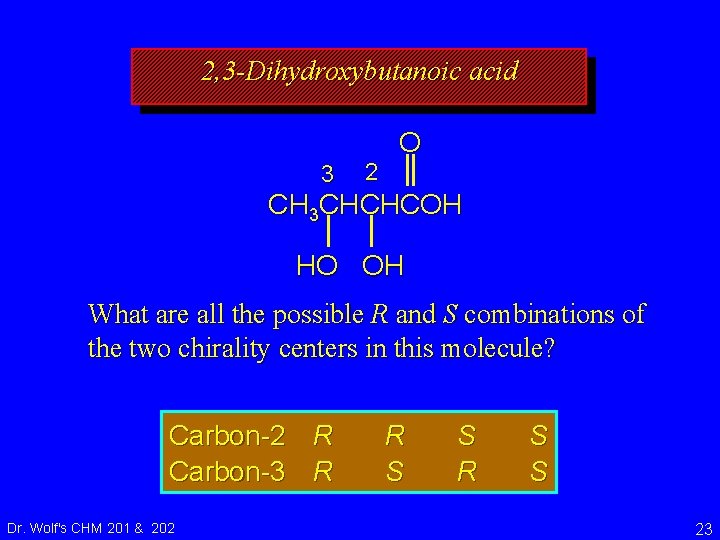
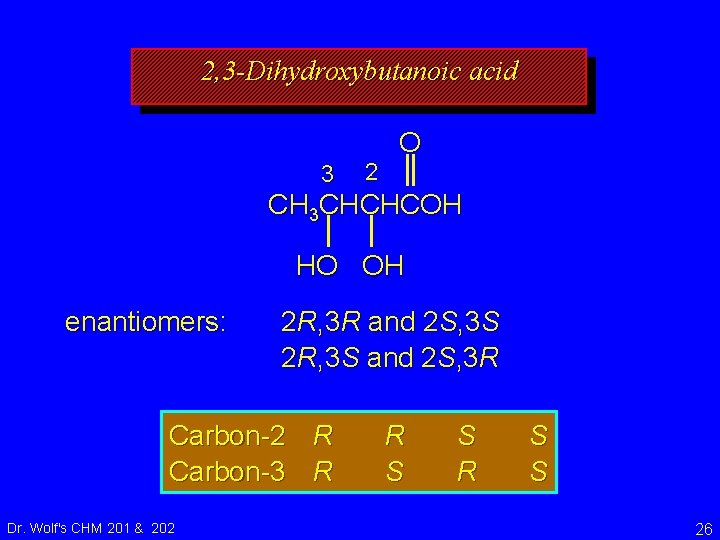
2, 3 -Dihydroxybutanoic acid 3 2 O CH 3 CHCHCOH HO OH What are all the possible R and S combinations of the two chirality centers in this molecule? Dr. Wolf's CHM 201 & 202 22

2, 3 -Dihydroxybutanoic acid 3 2 O CH 3 CHCHCOH HO OH What are all the possible R and S combinations of the two chirality centers in this molecule? Carbon-2 R Carbon-3 R Dr. Wolf's CHM 201 & 202 R S S 23

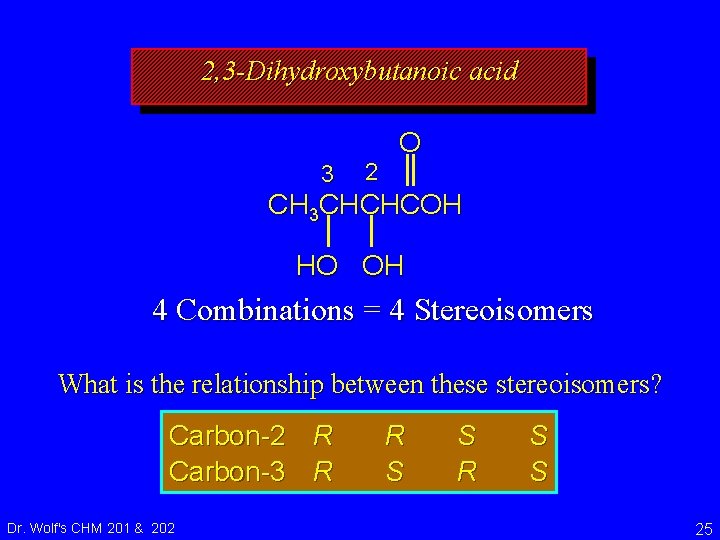
2, 3 -Dihydroxybutanoic acid 3 2 O CH 3 CHCHCOH HO OH 4 Combinations = 4 Stereoisomers Carbon-2 R Carbon-3 R Dr. Wolf's CHM 201 & 202 R S S 24

2, 3 -Dihydroxybutanoic acid 3 2 O CH 3 CHCHCOH HO OH 4 Combinations = 4 Stereoisomers What is the relationship between these stereoisomers? Carbon-2 R Carbon-3 R Dr. Wolf's CHM 201 & 202 R S S 25

2, 3 -Dihydroxybutanoic acid 3 2 O CH 3 CHCHCOH HO OH enantiomers: 2 R, 3 R and 2 S, 3 S 2 R, 3 S and 2 S, 3 R Carbon-2 R Carbon-3 R Dr. Wolf's CHM 201 & 202 R S S 26
![CO 2 H HO H a 9 5 CO 2 H a CO 2 H HO H [a] = -9. 5° CO 2 H [a] =](https://slidetodoc.com/presentation_image_h2/bd07b7a33d2b594bfa1e589bf3782ccd/image-27.jpg)
CO 2 H HO H [a] = -9. 5° CO 2 H [a] = +9. 5° R H OH R enantiomers H HO S H S CH 3 CO 2 H HO HO S R H H S CH 3 OH Dr. Wolf's CHM 201 & 202 enantiomers [a] = +17. 8° [a] = -17. 8° OH H H OH R CH 3 27

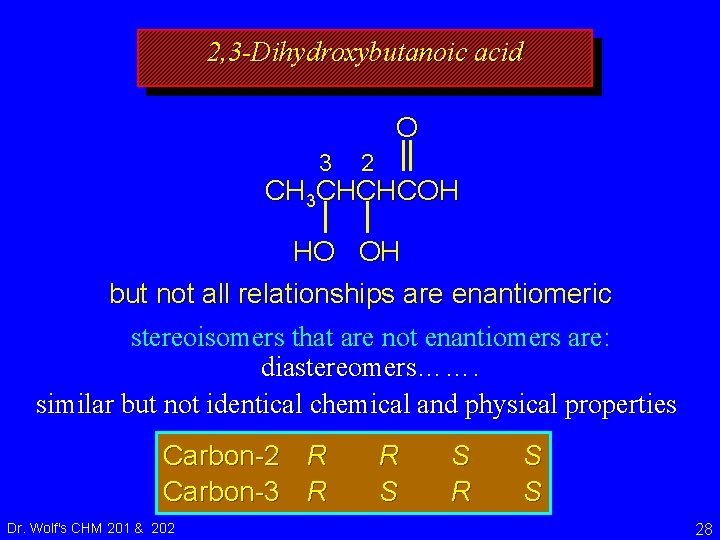
2, 3 -Dihydroxybutanoic acid O 3 2 CH 3 CHCHCOH HO OH but not all relationships are enantiomeric stereoisomers that are not enantiomers are: diastereomers……. similar but not identical chemical and physical properties Carbon-2 R Carbon-3 R Dr. Wolf's CHM 201 & 202 R S S 28

Isomers constitutional isomers enantiomers Dr. Wolf's CHM 201 & 202 stereoisomers diastereomers 29
![CO 2 H HO H a 9 5 CO 2 H a CO 2 H HO H [a] = -9. 5° CO 2 H [a] =](https://slidetodoc.com/presentation_image_h2/bd07b7a33d2b594bfa1e589bf3782ccd/image-30.jpg)
CO 2 H HO H [a] = -9. 5° CO 2 H [a] = +9. 5° R H OH R enantiomers H HO S H S CH 3 diastereomers CO 2 H HO HO S R H H S CH 3 OH Dr. Wolf's CHM 201 & 202 enantiomers [a] = +17. 8° [a] = -17. 8° OH H H OH R CH 3 30

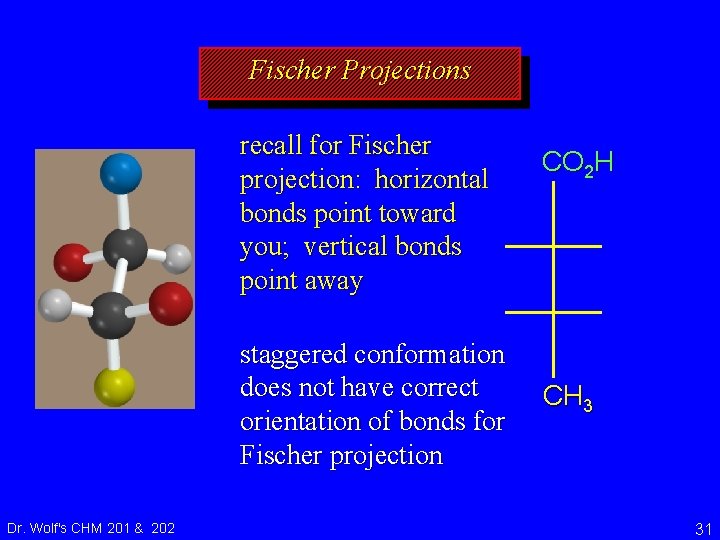
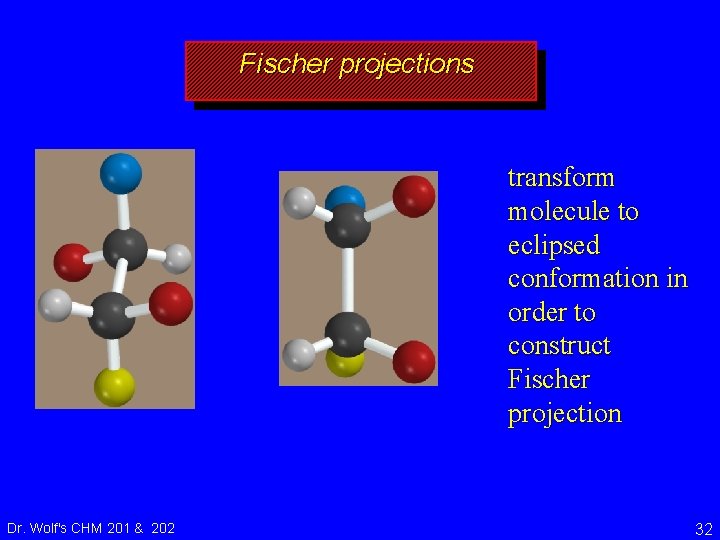
Fischer Projections recall for Fischer projection: horizontal bonds point toward you; vertical bonds point away staggered conformation does not have correct orientation of bonds for Fischer projection Dr. Wolf's CHM 201 & 202 CO 2 H CH 3 31

Fischer projections transform molecule to eclipsed conformation in order to construct Fischer projection Dr. Wolf's CHM 201 & 202 32

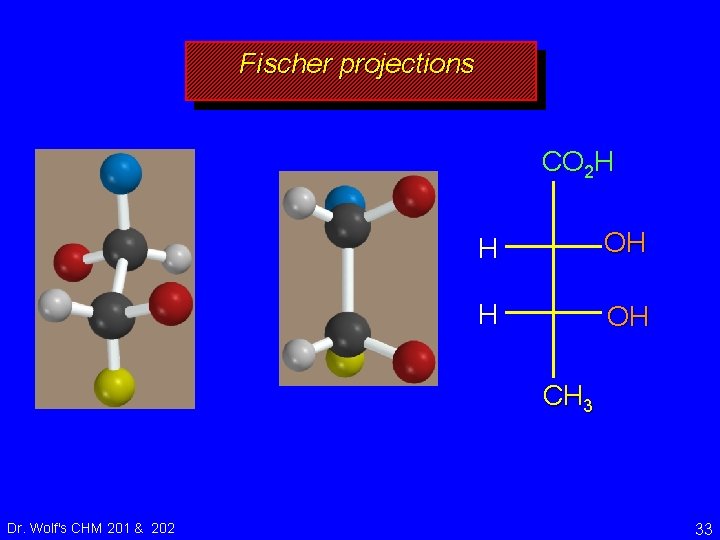
Fischer projections CO 2 H H OH CH 3 Dr. Wolf's CHM 201 & 202 33

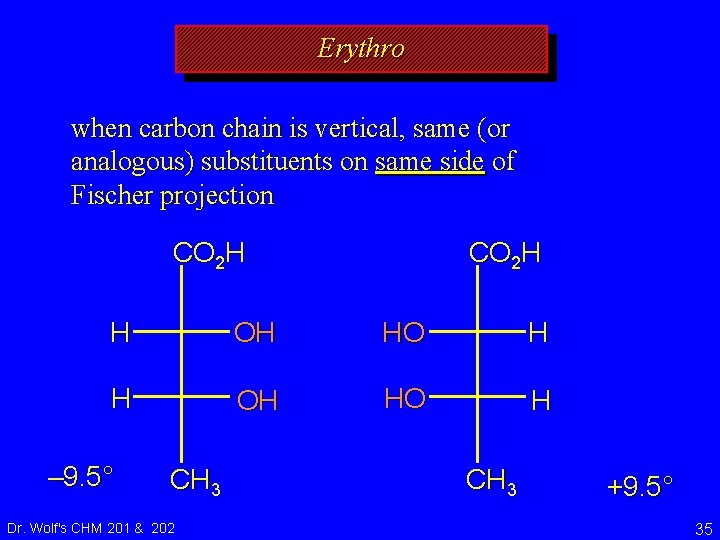
Erythro and Threo stereochemical prefixes used to specify relative configuration in molecules with two chirality centers easiest to apply using Fischer projections orientation: vertical carbon chain Dr. Wolf's CHM 201 & 202 34

Erythro when carbon chain is vertical, same (or analogous) substituents on same side of Fischer projection CO 2 H H OH HO H – 9. 5° CH 3 Dr. Wolf's CHM 201 & 202 CH 3 +9. 5° 35

Threo when carbon chain is vertical, same (or analogous) substituents on opposite sides of Fischer projection CO 2 H OH H HO +17. 8° H CH 3 Dr. Wolf's CHM 201 & 202 CO 2 H H HO OH H CH 3 – 17. 8° 36

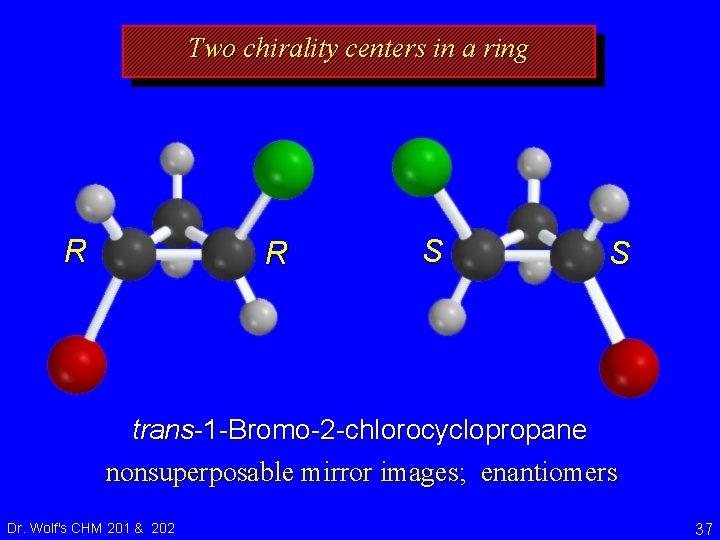
Two chirality centers in a ring R R S S trans-1 -Bromo-2 -chlorocyclopropane nonsuperposable mirror images; enantiomers Dr. Wolf's CHM 201 & 202 37

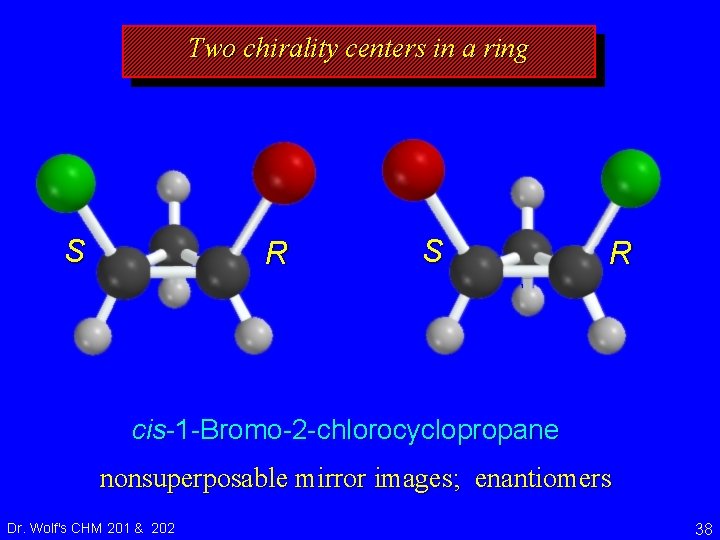
Two chirality centers in a ring S R cis-1 -Bromo-2 -chlorocyclopropane nonsuperposable mirror images; enantiomers Dr. Wolf's CHM 201 & 202 38

Two chirality centers in a ring S R cis-1 -Bromo-2 -chlorotrans-1 -Bromo-2 -chlorocyclopropane stereoisomers that are not enantiomers; diastereomers Dr. Wolf's CHM 201 & 202 39

7. 11 Achiral Molecules with Two Chirality Centers It is possible for a molecule to have chirality centers yet be achiral. Dr. Wolf's CHM 201 & 202 40

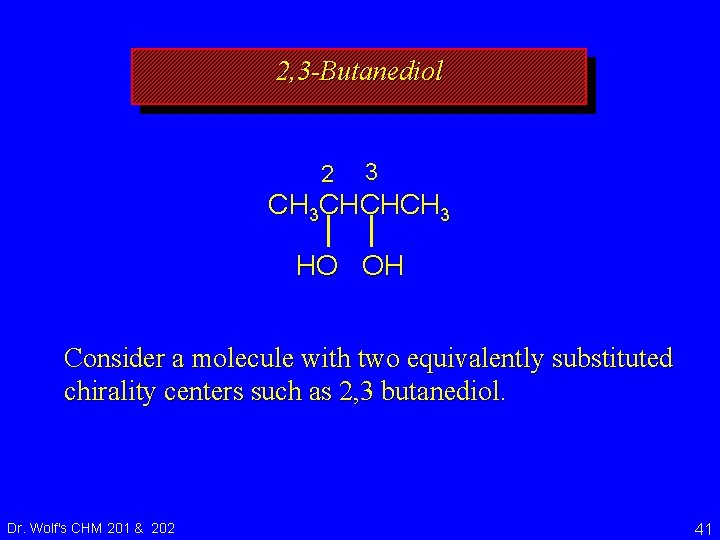
2, 3 -Butanediol 2 3 CH 3 CHCHCH 3 HO OH Consider a molecule with two equivalently substituted chirality centers such as 2, 3 butanediol. Dr. Wolf's CHM 201 & 202 41

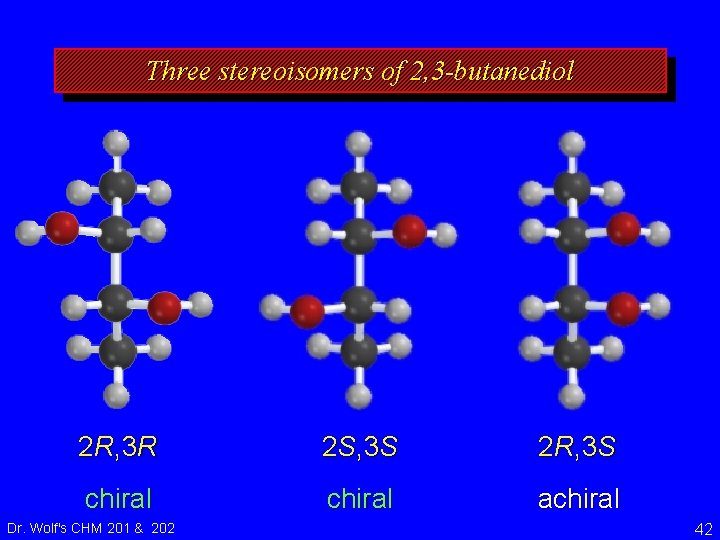
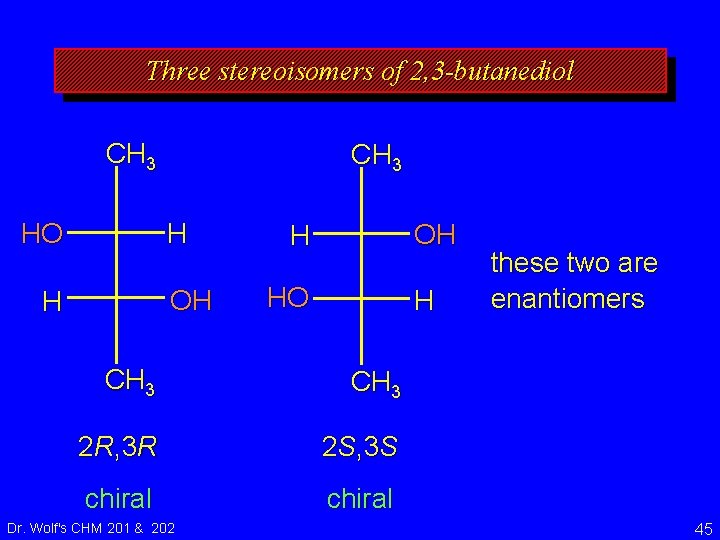
Three stereoisomers of 2, 3 -butanediol 2 R, 3 R 2 S, 3 S 2 R, 3 S chiral achiral Dr. Wolf's CHM 201 & 202 42

Three stereoisomers of 2, 3 -butanediol CH 3 H HO OH H H HO CH 3 OH H H OH CH 3 2 R, 3 R 2 S, 3 S 2 R, 3 S chiral achiral Dr. Wolf's CHM 201 & 202 43

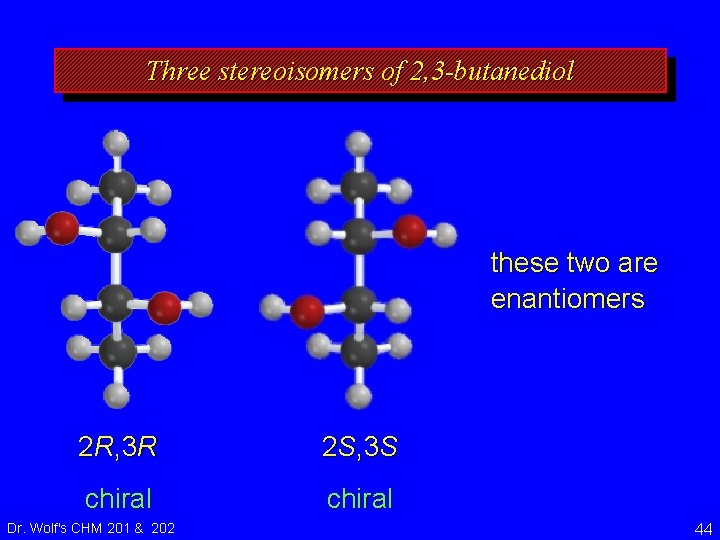
Three stereoisomers of 2, 3 -butanediol these two are enantiomers 2 R, 3 R 2 S, 3 S chiral Dr. Wolf's CHM 201 & 202 44

Three stereoisomers of 2, 3 -butanediol CH 3 H HO OH H HO H CH 3 2 R, 3 R 2 S, 3 S chiral Dr. Wolf's CHM 201 & 202 these two are enantiomers 45

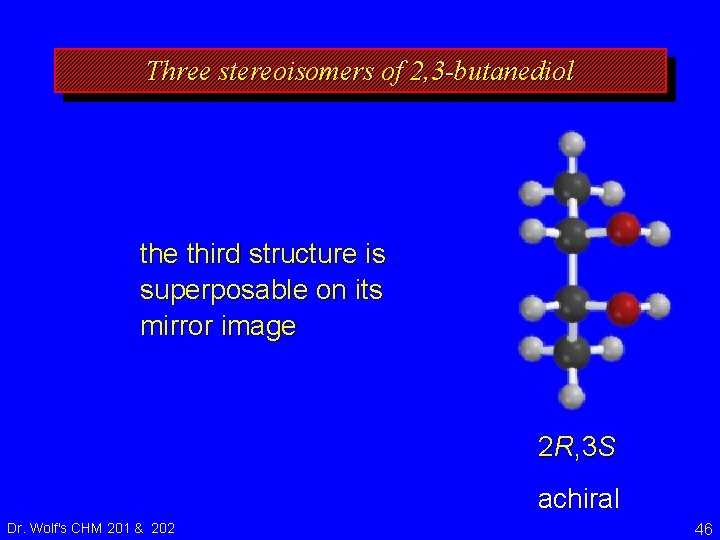
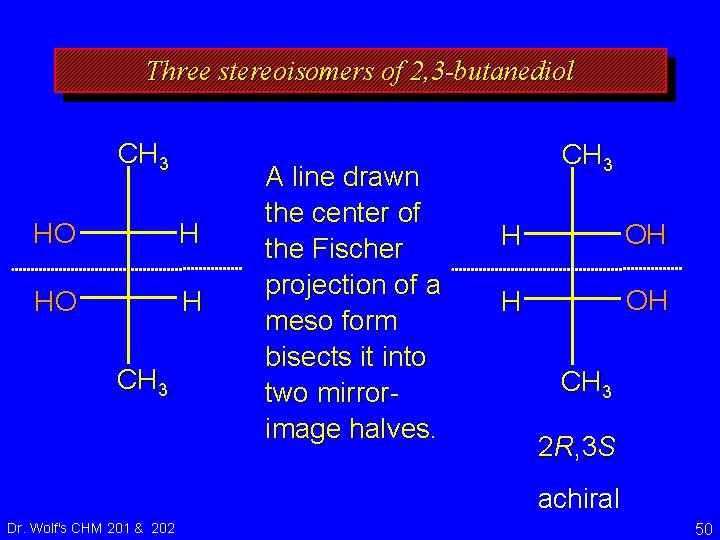
Three stereoisomers of 2, 3 -butanediol the third structure is superposable on its mirror image 2 R, 3 S achiral Dr. Wolf's CHM 201 & 202 46

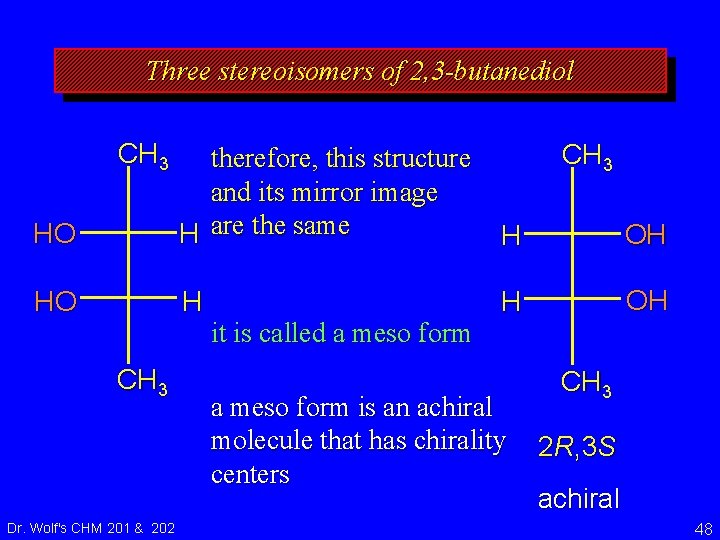
Three stereoisomers of 2, 3 -butanediol therefore, this structure and its mirror image are the same it is called a meso form is an achiral molecule that has chirality centers Dr. Wolf's CHM 201 & 202 2 R, 3 S achiral 47

Three stereoisomers of 2, 3 -butanediol CH 3 HO therefore, this structure and its mirror image H are the same H OH HO H H OH CH 3 Dr. Wolf's CHM 201 & 202 it is called a meso form is an achiral molecule that has chirality centers CH 3 2 R, 3 S achiral 48

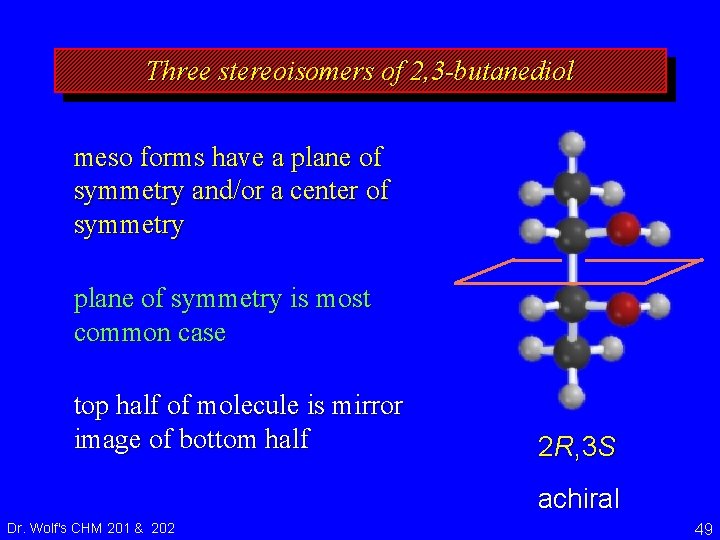
Three stereoisomers of 2, 3 -butanediol meso forms have a plane of symmetry and/or a center of symmetry plane of symmetry is most common case top half of molecule is mirror image of bottom half 2 R, 3 S achiral Dr. Wolf's CHM 201 & 202 49

Three stereoisomers of 2, 3 -butanediol CH 3 HO H CH 3 A line drawn the center of the Fischer projection of a meso form bisects it into two mirrorimage halves. CH 3 H OH CH 3 2 R, 3 S achiral Dr. Wolf's CHM 201 & 202 50

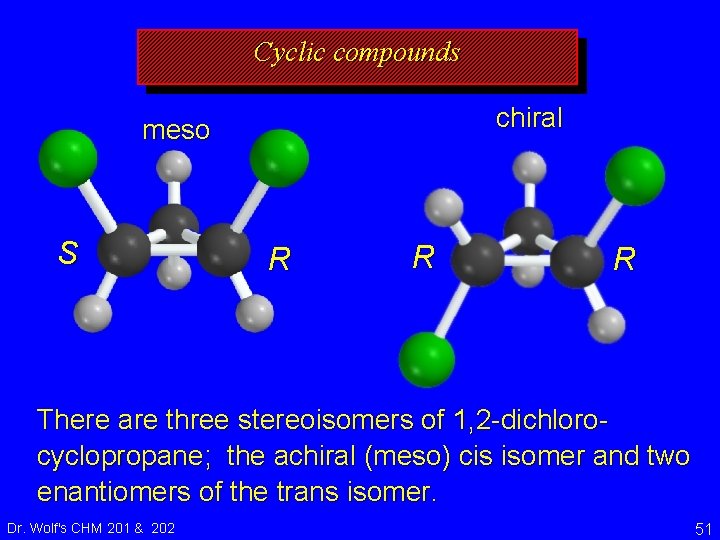
Cyclic compounds chiral meso S R R R There are three stereoisomers of 1, 2 -dichlorocyclopropane; the achiral (meso) cis isomer and two enantiomers of the trans isomer. Dr. Wolf's CHM 201 & 202 51
 Wedge dash to fischer
Wedge dash to fischer Fischer projection of galactose
Fischer projection of galactose Chapter 5 selecting a topic and a purpose
Chapter 5 selecting a topic and a purpose Sentence purpose
Sentence purpose Síntesis de williamson
Síntesis de williamson Karl fischer titration formula
Karl fischer titration formula Acid anhydride and alcohol
Acid anhydride and alcohol Acidity of carboxylic acid derivatives
Acidity of carboxylic acid derivatives Gott fischer wetten dass
Gott fischer wetten dass Pessi fischer
Pessi fischer Marietta fischer
Marietta fischer Draw an aldohexose.
Draw an aldohexose. Dr guy lustig - centre médico-esthétique séléstat
Dr guy lustig - centre médico-esthétique séléstat Manuela fischer eth
Manuela fischer eth Ala fischer ne icat etmiştir
Ala fischer ne icat etmiştir Acido 3 bromopropanoico
Acido 3 bromopropanoico Fischer tropsch
Fischer tropsch Fisher effect formula
Fisher effect formula R and s configuration fischer projection
R and s configuration fischer projection Chiral molecules
Chiral molecules Fischer quimica
Fischer quimica Lotta fischer
Lotta fischer Krista fischer
Krista fischer Fisher effect equation
Fisher effect equation General contractor and supplier
General contractor and supplier Glucose cyclic structure
Glucose cyclic structure Emil hermann fischer
Emil hermann fischer Melitta fischer-kern
Melitta fischer-kern Sifat laba ekonomi menurut fischer
Sifat laba ekonomi menurut fischer Lisbeth fischer
Lisbeth fischer Designating handedness using fischer projection formulas
Designating handedness using fischer projection formulas Marcelo rivano fischer
Marcelo rivano fischer Kristi simmons
Kristi simmons Alanine fischer projection
Alanine fischer projection D-aldopentose fischer projection
D-aldopentose fischer projection Fisher hsa
Fisher hsa Ruben hopwood
Ruben hopwood Order of reactivity of carboxylic acid derivatives
Order of reactivity of carboxylic acid derivatives Melitta fischer-kern
Melitta fischer-kern Polyhydroxylated aldehydes
Polyhydroxylated aldehydes Neopiagetians are
Neopiagetians are Lorenz lloyd fischer
Lorenz lloyd fischer Flandreau middle school
Flandreau middle school Fisher effect
Fisher effect Specific rotation units
Specific rotation units Fischer esterification methyl salicylate
Fischer esterification methyl salicylate Vukasin fischer
Vukasin fischer Kurt fischer theory
Kurt fischer theory Aminosure
Aminosure Tomasz landsberg
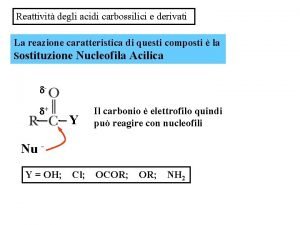
Tomasz landsberg Sostituzione nucleofila acilica
Sostituzione nucleofila acilica Hp fischer verpackungen
Hp fischer verpackungen