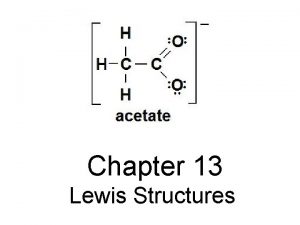
6 2 Lewis Structures Lewis Structuresshow arrangement of






- Slides: 16

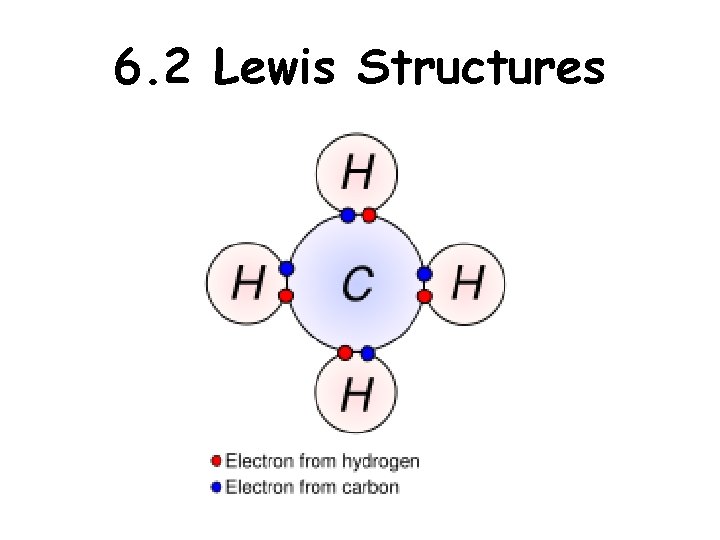
6. 2 Lewis Structures

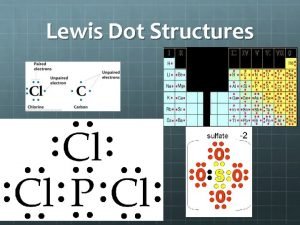
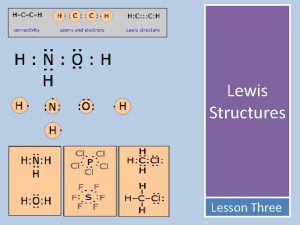
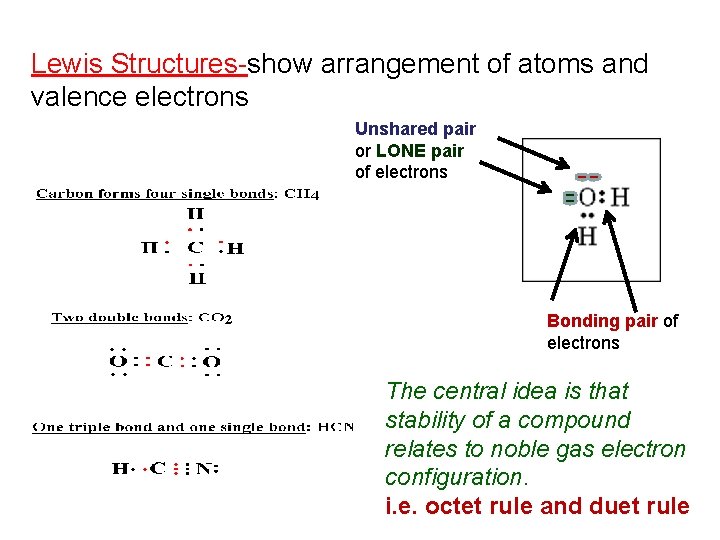
Lewis Structures-show arrangement of atoms and valence electrons Unshared pair or LONE pair of electrons Bonding pair of electrons The central idea is that stability of a compound relates to noble gas electron configuration. i. e. octet rule and duet rule

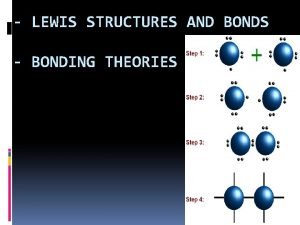
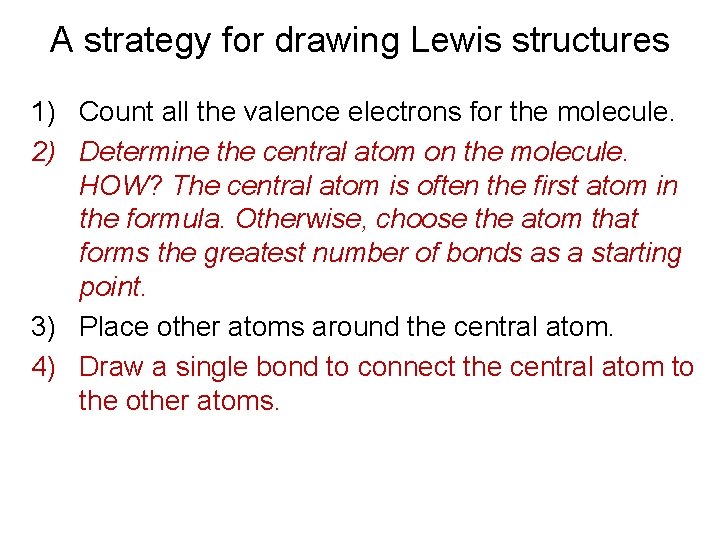
A strategy for drawing Lewis structures 1) Count all the valence electrons for the molecule. 2) Determine the central atom on the molecule. HOW? The central atom is often the first atom in the formula. Otherwise, choose the atom that forms the greatest number of bonds as a starting point. 3) Place other atoms around the central atom. 4) Draw a single bond to connect the central atom to the other atoms.

Strategy continued 5) Add the remaining electrons to the atoms to satisfy the octet rule (except for hydrogen which only needs 2 electrons). 6) Double check that you used the right number of electrons and the octet rule is followed. 7) If the central atom still does not achieve an octet, and it is expected to do so, move lone pairs from the outlying atoms to form a double or triple bond between atoms.

Helpful hints HONC rule: generally H bonds to 1 other atom (1 valence electron- needs 1 more) O bonds to 2 other atoms (has 6 valence electron-needs 2 more) N bonds to 3 other atoms (has 5 valence electrons- needs 3 more) C bonds to 4 other atoms (has 4 valence electrons-needs 4 more) Carbon, nitrogen and oxygen commonly form double bonds. Hydrogen, and the halogens usually share one Hydrogen pair of electrons (no double or triple bonds) Nitrogen and carbon can share three pairs of electrons to form a triple bond.

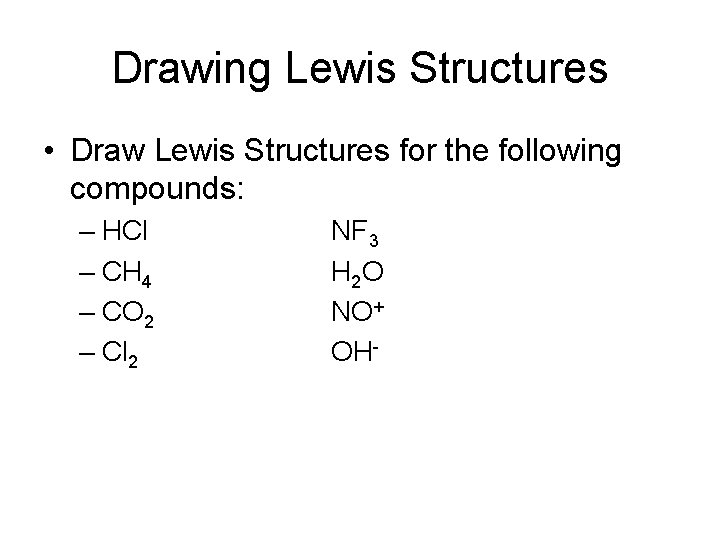
Drawing Lewis Structures • Draw Lewis Structures for the following compounds: – HCl – CH 4 – CO 2 – Cl 2 NF 3 H 2 O NO+ OH-

Lewis Structures • Draw Lewis Structures for the following compounds: – O 2 – C 2 H 4 N 2 HCN C 2 H 2 • Covalent bonds may be classified as single, double, or triple depending on the number of pairs of electrons shared.

Some molecules can be represented by more than one Lewis structure • They are called resonance structures. • Draw the structure for SO 2 • The actual structure is an average of the resonance structures. • Experiments show that the two bonds are really of equal length and strength. The bond length is shorter than a single bond, but longer than a double bond.

Drawing Resonance Structures • Draw Lewis Structures for the following compounds – NO 2 -1 – SO 3 – O 3

6. 2 Writing Formulas & Naming Covalent Compounds 1. IONIC compounds are made from 2 ions (metal + nonmetal) 2. COVALENT compounds are made from two or more non-metals. ** Have different methods for writing formulas and naming.

6. 2 Naming Covalent Compounds • Nonmetal + nonmetals can form more than one compound with each other … Ex) NO, NO 2, NO 5 • Cannot use Ionic rules … otherwise, all these would all have the same name “Nitrogen Oxide”

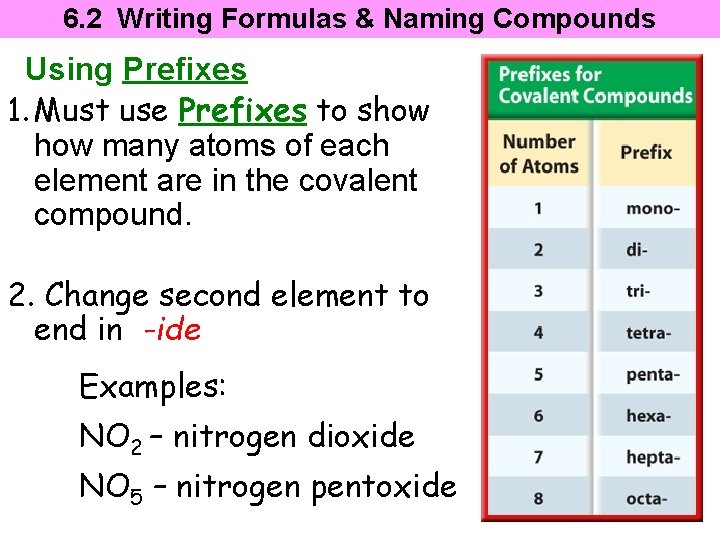
6. 2 Writing Formulas & Naming Compounds Using Prefixes 1. Must use Prefixes to show many atoms of each element are in the covalent compound. 2. Change second element to end in -ide Examples: NO 2 – nitrogen dioxide NO 5 – nitrogen pentoxide

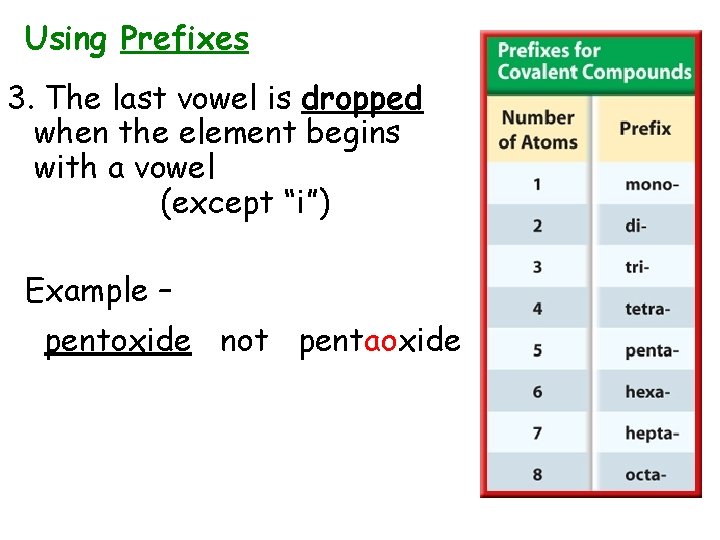
Using Prefixes 3. The last vowel is dropped when the element begins with a vowel (except “i”) Example – pentoxide not pentaoxide

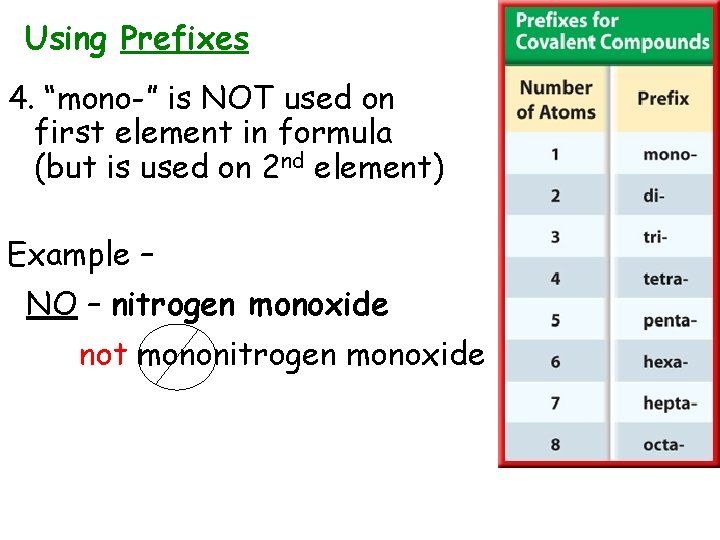
Using Prefixes 4. “mono-” is NOT used on first element in formula (but is used on 2 nd element) Example – NO – nitrogen monoxide not mononitrogen monoxide

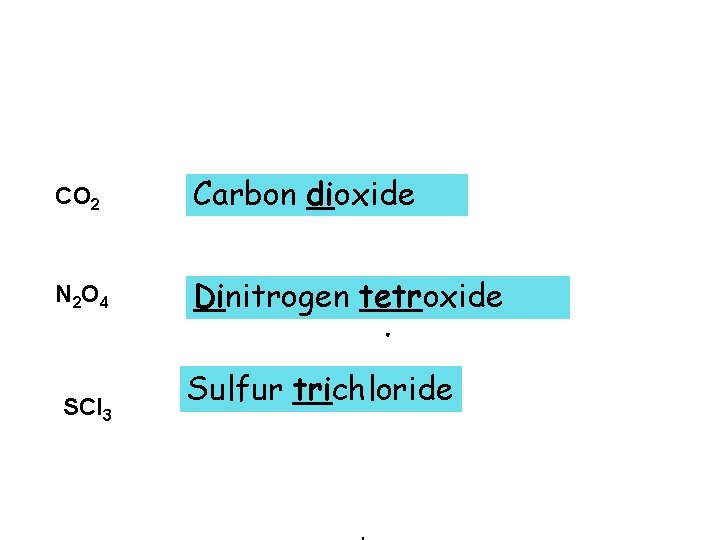
CO 2 Carbon dioxide N 2 O 4 Dinitrogen tetroxide SCl 3 Sulfur trichloride

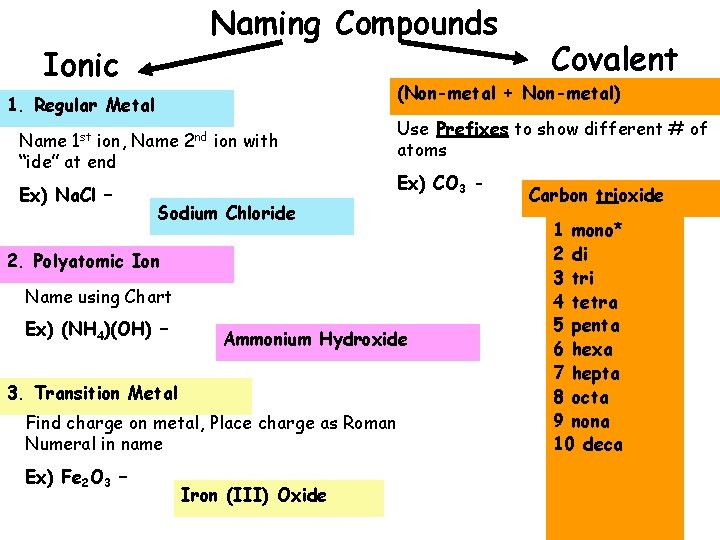
Naming Compounds Ionic (Non-metal + Non-metal) 1. Regular Metal Name 1 st ion, Name 2 nd ion with “ide” at end Ex) Na. Cl – Use Prefixes to show different # of atoms Ex) CO 3 - Sodium Chloride 2. Polyatomic Ion Name using Chart Ex) (NH 4)(OH) – Ammonium Hydroxide 3. Transition Metal Find charge on metal, Place charge as Roman Numeral in name Ex) Fe 2 O 3 – Covalent Iron (III) Oxide Carbon trioxide 1 mono* 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca
 Examples of homologous
Examples of homologous No+ vsepr
No+ vsepr Expanded octet lewis structure
Expanded octet lewis structure Lewis structures cannot
Lewis structures cannot Rules for drawing lewis structures
Rules for drawing lewis structures Formal charge of hcn
Formal charge of hcn Lewis structure of pf3
Lewis structure of pf3 Draw a bohr diagram for hydrogen and neon
Draw a bohr diagram for hydrogen and neon Dicarbon dihydride lewis dot structure
Dicarbon dihydride lewis dot structure Lewis dot diagram
Lewis dot diagram Lewis structures represent the
Lewis structures represent the Pcl lewis dot structure
Pcl lewis dot structure Lewis dot structure cl
Lewis dot structure cl Energy of solid, liquid and gas
Energy of solid, liquid and gas Arrangement of electrons
Arrangement of electrons Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Diplomatic protocol seating arrangement
Diplomatic protocol seating arrangement