5 th Paris Hepatitis C Conference Paris 30




- Slides: 15

5 th Paris Hepatitis C Conference Paris, 30 January 2012 Luncheon: How to optimize treatment of G 2 and G 3 patients Alessio Aghemo First Division of Gastroenterology Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico Università degli Studi di Milano

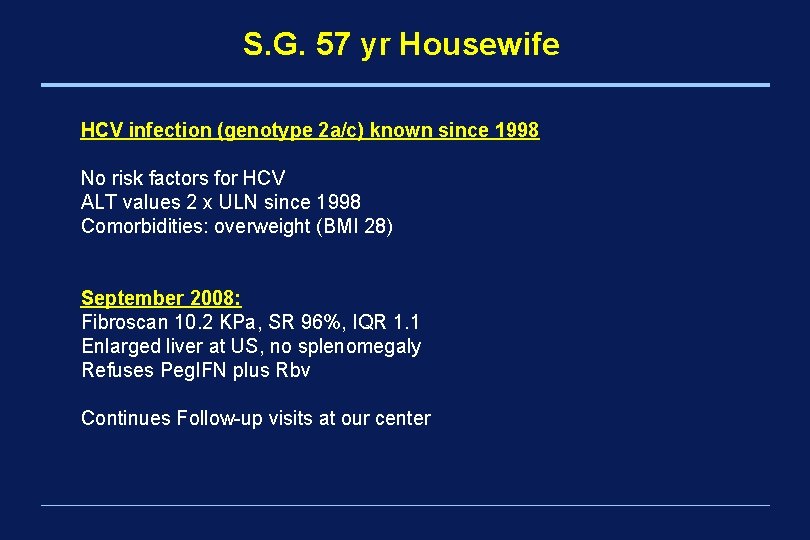
S. G. 57 yr Housewife HCV infection (genotype 2 a/c) known since 1998 No risk factors for HCV ALT values 2 x ULN since 1998 Comorbidities: overweight (BMI 28) September 2008: Fibroscan 10. 2 KPa, SR 96%, IQR 1. 1 Enlarged liver at US, no splenomegaly Refuses Peg. IFN plus Rbv Continues Follow-up visits at our center

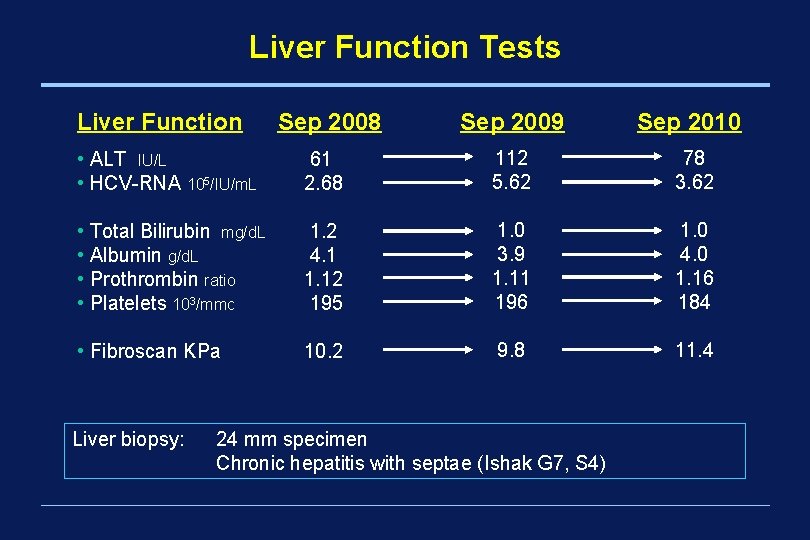
Liver Function Tests Liver Function Sep 2008 Sep 2009 Sep 2010 • ALT IU/L • HCV-RNA 105/IU/m. L 61 2. 68 112 5. 62 78 3. 62 • Total Bilirubin mg/d. L • Albumin g/d. L • Prothrombin ratio • Platelets 103/mmc 1. 2 4. 1 1. 12 195 1. 0 3. 9 1. 11 196 1. 0 4. 0 1. 16 184 • Fibroscan KPa 10. 2 9. 8 11. 4 Liver biopsy: 24 mm specimen Chronic hepatitis with septae (Ishak G 7, S 4)

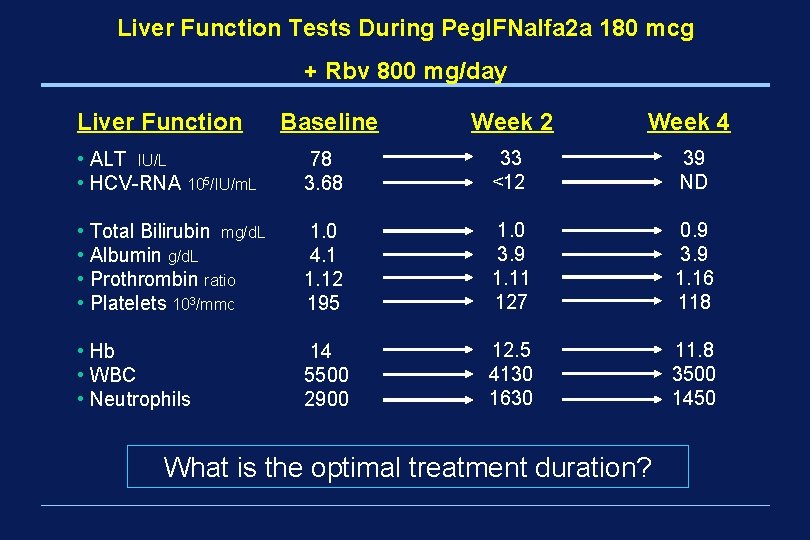
Liver Function Tests During Peg. IFNalfa 2 a 180 mcg + Rbv 800 mg/day Liver Function Baseline Week 2 Week 4 • ALT IU/L • HCV-RNA 105/IU/m. L 78 3. 68 33 <12 39 ND • Total Bilirubin mg/d. L • Albumin g/d. L • Prothrombin ratio • Platelets 103/mmc 1. 0 4. 1 1. 12 195 1. 0 3. 9 1. 11 127 0. 9 3. 9 1. 16 118 • Hb • WBC • Neutrophils 14 5500 2900 12. 5 4130 1630 11. 8 3500 1450 What is the optimal treatment duration?

HCV RNA Kinetics During Peg. IFN + Rbv to Predict Treatment Outcome and Individualize Treatment Duration HCV-2/3 Patients Weeks Peg. IFNalfa + RBV Peg. IFNalfa 800/day + RBV 800 mg/day 0 12 -16 24

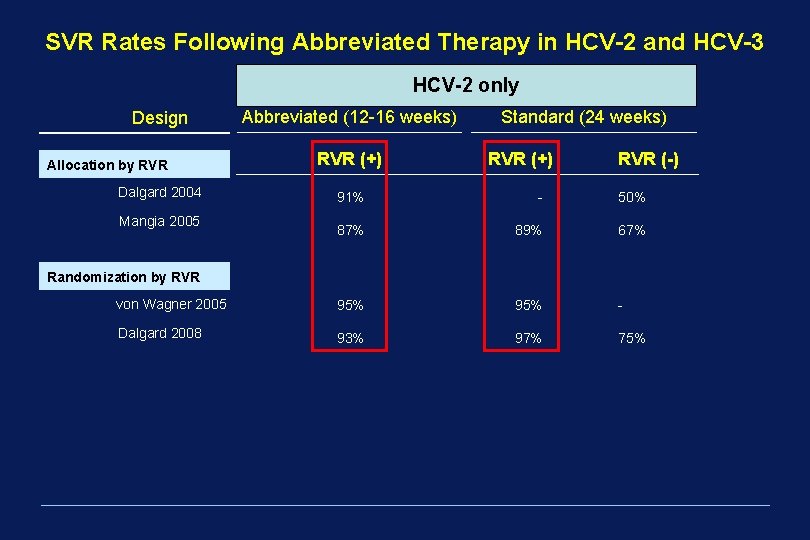
SVR Rates Following Abbreviated Therapy in HCV-2 and HCV-3 HCV-2 only Design Allocation by RVR Abbreviated (24 weeks)RVR (LOD) No. (12 -16 weeks) Regimen Standard. Duration RVR (+) Dalgard 2004 122 91% Peg 2 b + Rbv wbd Mangia 2005 283 87% Peg 2 b 1. 0 + Rbv wbd RVR (-) - 14 50% w 78% (50 IU) 89% 12 w 67% 63% (50 IU) Randomization by RVR von Wagner 2005 153 95% Peg 2 a + Rbv wbd 95% 16 -w 93% (600 IU) Dalgard 2008 428 93% Peg 2 b + Rbv wbd 97% 14 75% w 71% (50 IU) Baseline Randomization Shiffman 2007 1469 Peg 2 a + Rbv 800 mg 16 w 65% (50 IU) Lagging 2008 382 Peg 2 a + Rbv 800 mg 12 w 60% (15 IU)

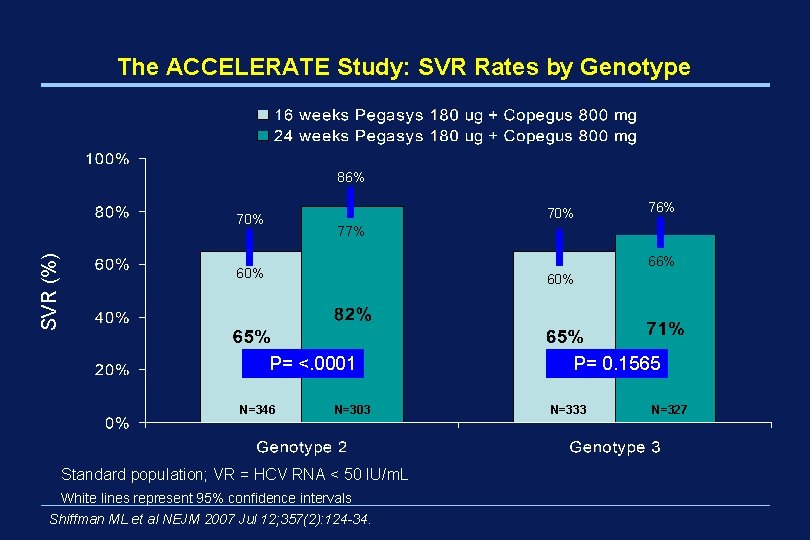
The ACCELERATE Study: SVR Rates by Genotype 86% 70% SVR (%) 70% 76% 77% 66% 60% P= <. 0001 N=346 N=303 Standard population; VR = HCV RNA < 50 IU/m. L White lines represent 95% confidence intervals Shiffman ML et al NEJM 2007 Jul 12; 357(2): 124 -34. P= 0. 1565 N=333 N=327

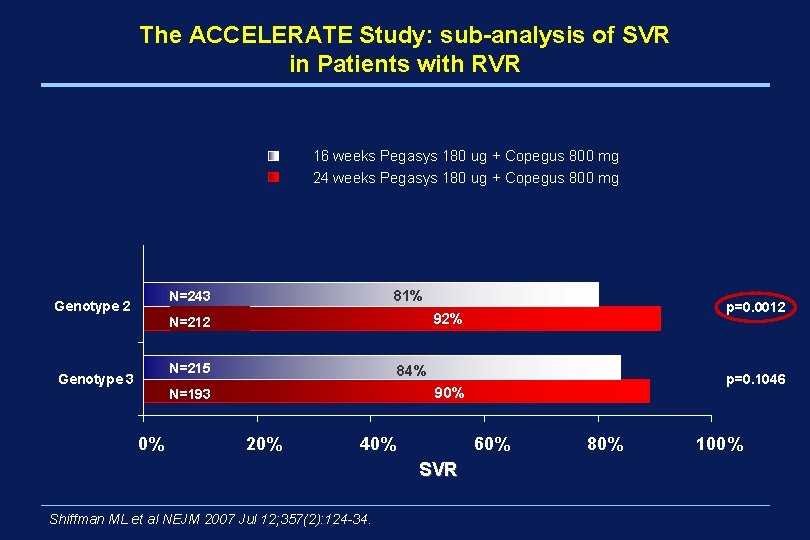
The ACCELERATE Study: sub-analysis of SVR in Patients with RVR 16 weeks Pegasys 180 ug + Copegus 800 mg 24 weeks Pegasys 180 ug + Copegus 800 mg 81% N=243 Genotype 2 92% N=212 N=215 Genotype 3 84% p=0. 1046 90% N=193 0% p=0. 0012 20% 40% 60% SVR Shiffman ML et al NEJM 2007 Jul 12; 357(2): 124 -34. 80% 100%

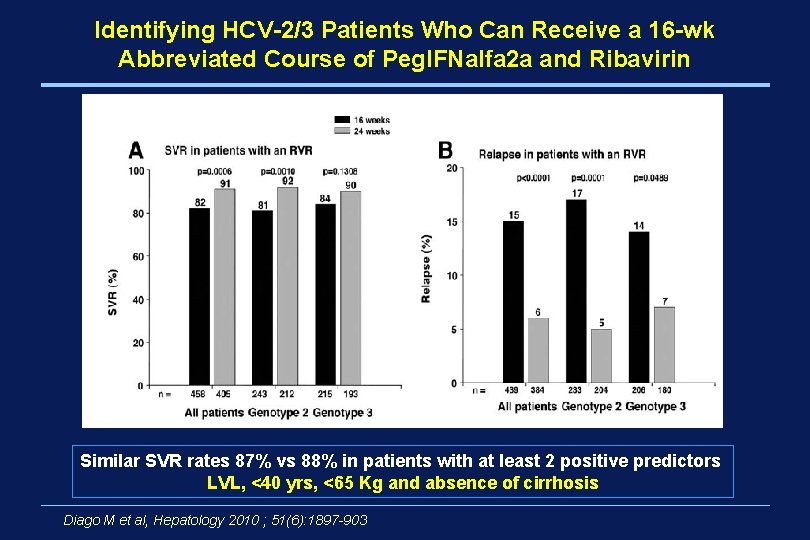
Identifying HCV-2/3 Patients Who Can Receive a 16 -wk Abbreviated Course of Peg. IFNalfa 2 a and Ribavirin Similar SVR rates 87% vs 88% in patients with at least 2 positive predictors LVL, <40 yrs, <65 Kg and absence of cirrhosis Diago M et al, Hepatology 2010 ; 51(6): 1897 -903

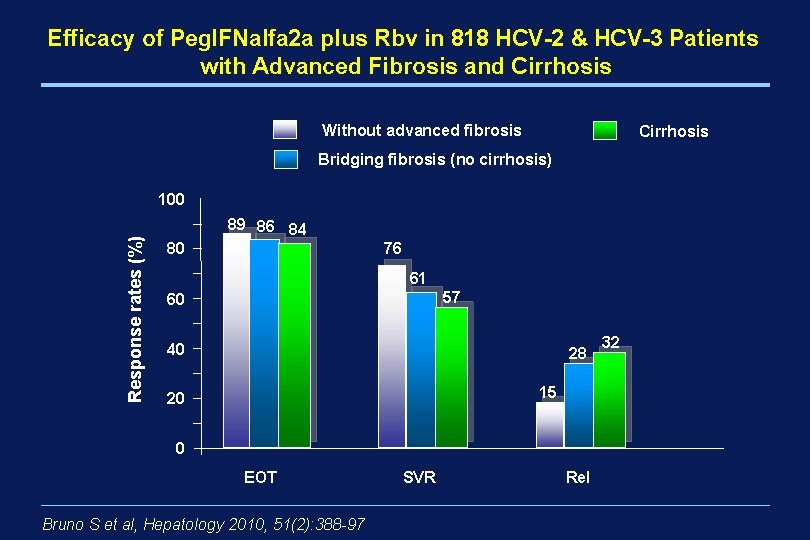
Efficacy of Peg. IFNalfa 2 a plus Rbv in 818 HCV-2 & HCV-3 Patients with Advanced Fibrosis and Cirrhosis Without advanced fibrosis Cirrhosis Bridging fibrosis (no cirrhosis) Response rates (%) 100 89 86 84 76 80 61 57 60 40 28 15 20 0 EOT Bruno S et al, Hepatology 2010, 51(2): 388 -97 SVR Rel 32

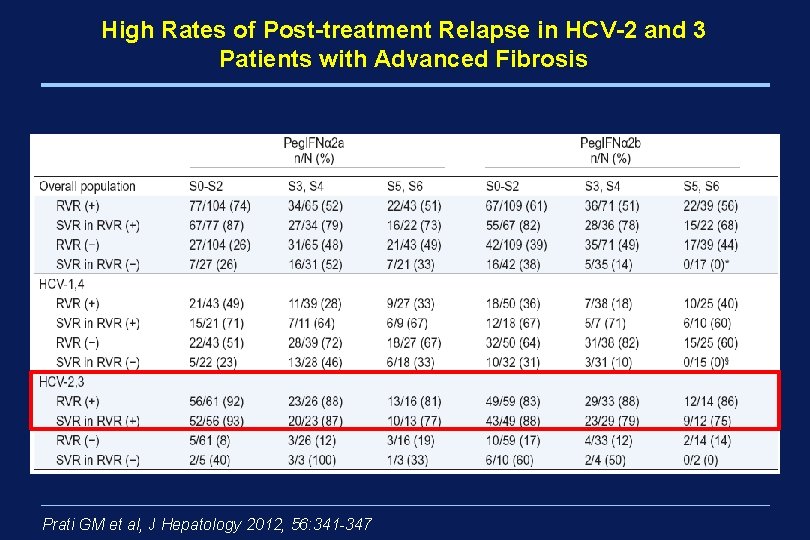
High Rates of Post-treatment Relapse in HCV-2 and 3 Patients with Advanced Fibrosis Peg. IFNalfa 2 a + Rbv 800 mg Prati GM et al, J Hepatology 2012, 56: 341 -347 Peg. IFNalfa 2 b + Rbv 800 -1200 mg

High Rates of Post-treatment Relapse in HCV-2 and 3 Patients with Advanced Fibrosis Prati GM et al, J Hepatology 2012, 56: 341 -347

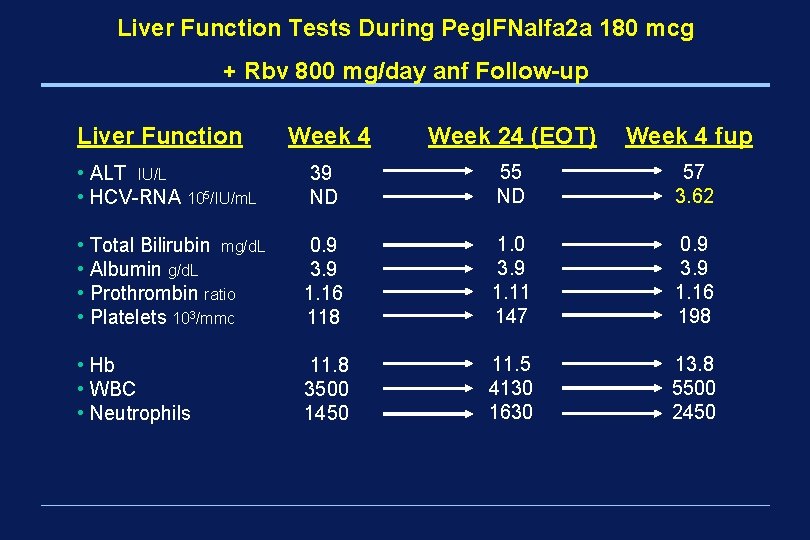
Liver Function Tests During Peg. IFNalfa 2 a 180 mcg + Rbv 800 mg/day anf Follow-up Liver Function Week 4 Week 24 (EOT) Week 4 fup • ALT IU/L • HCV-RNA 105/IU/m. L 39 ND 55 ND 57 3. 62 • Total Bilirubin mg/d. L • Albumin g/d. L • Prothrombin ratio • Platelets 103/mmc 0. 9 3. 9 1. 16 118 1. 0 3. 9 1. 11 147 0. 9 3. 9 1. 16 198 • Hb • WBC • Neutrophils 11. 8 3500 1450 11. 5 4130 1630 13. 8 5500 2450

What Now? ? ? § Retreat with Peg. IFN plus Rbv? § Retreat using high dose Rbv? (Off Label) § Retreat using Telaprevir? (Off Label) § Wait for new drugs? (2014 -2015)

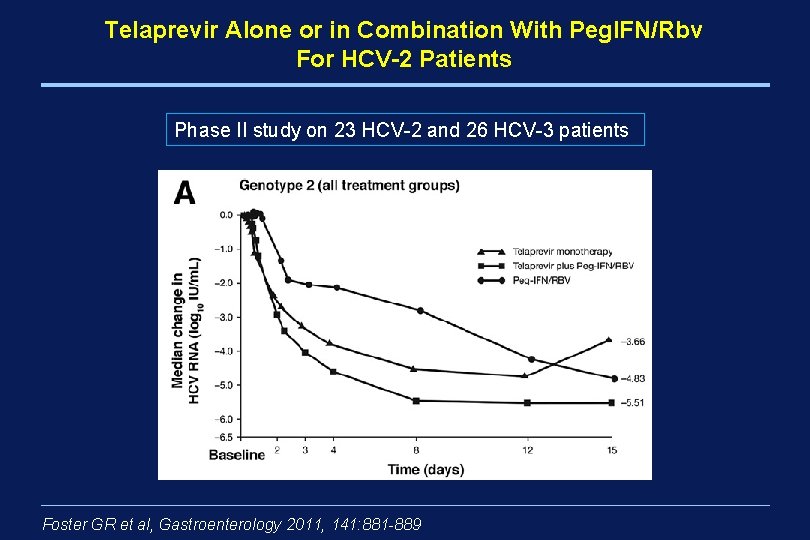
Telaprevir Alone or in Combination With Peg. IFN/Rbv For HCV-2 Patients Phase II study on 23 HCV-2 and 26 HCV-3 patients Foster GR et al, Gastroenterology 2011, 141: 881 -889
 Hepatitis viral
Hepatitis viral Hepatitis b vaccine series adults
Hepatitis b vaccine series adults Exudative ascites
Exudative ascites Papillomitosis
Papillomitosis Hepatitis e
Hepatitis e Hepatitis a treatment
Hepatitis a treatment Hepatitis b is a silent killer
Hepatitis b is a silent killer Hepatitis a
Hepatitis a Dosis de hepatitis b
Dosis de hepatitis b Hepatitis b panel
Hepatitis b panel Klasifikasi hepatitis a
Klasifikasi hepatitis a Hepatitis b transmission
Hepatitis b transmission Hepatitis d
Hepatitis d Hepatitis lupica
Hepatitis lupica Neonatal jaundice
Neonatal jaundice Window period of hepatitis b
Window period of hepatitis b