2014 Pearson Education Inc 2014 Pearson Education Inc


- Slides: 28

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

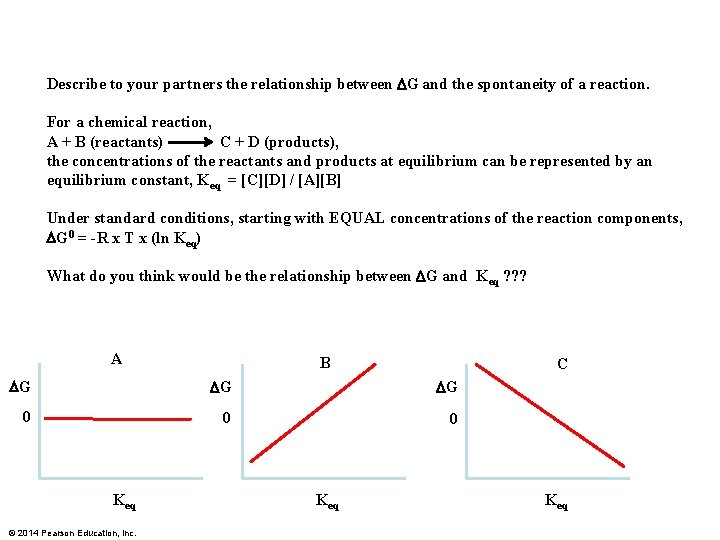
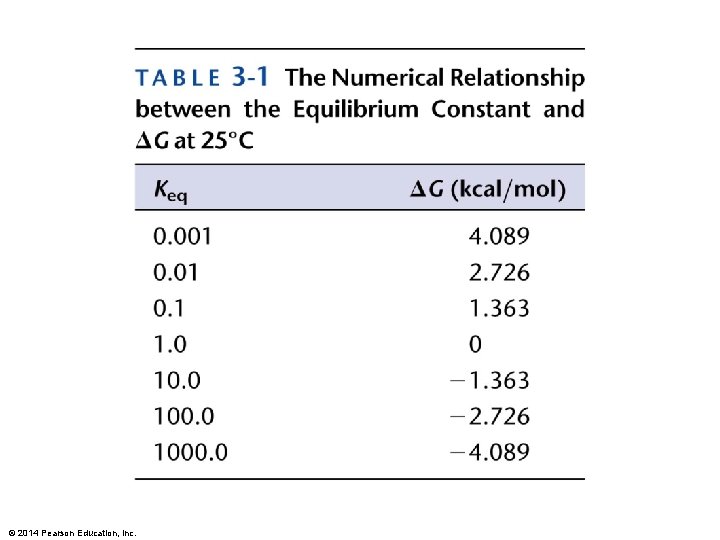
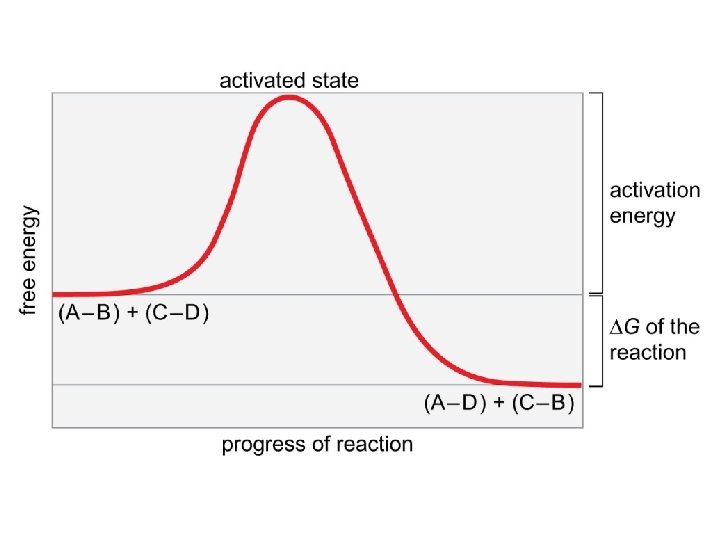
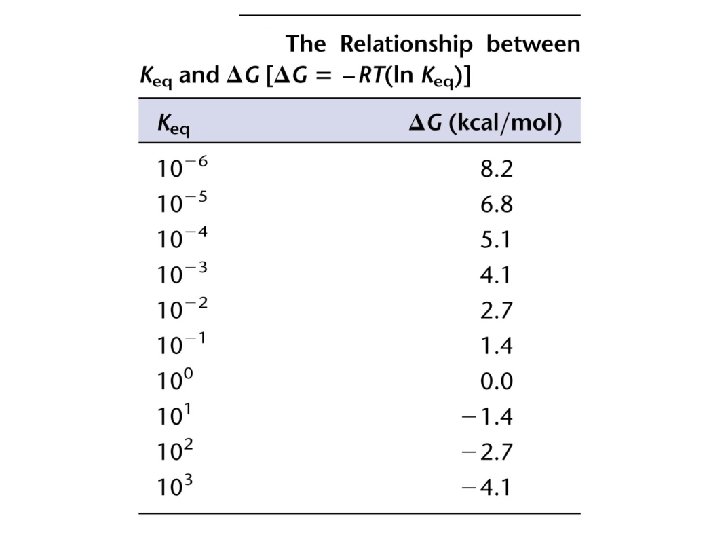
Describe to your partners the relationship between DG and the spontaneity of a reaction. For a chemical reaction, A + B (reactants) C + D (products), the concentrations of the reactants and products at equilibrium can be represented by an equilibrium constant, Keq = [C][D] / [A][B] Under standard conditions, starting with EQUAL concentrations of the reaction components, DG 0 = -R x T x (ln Keq) What do you think would be the relationship between DG and Keq ? ? ? A B C DG DG DG 0 0 0 Keq © 2014 Pearson Education, Inc. Keq

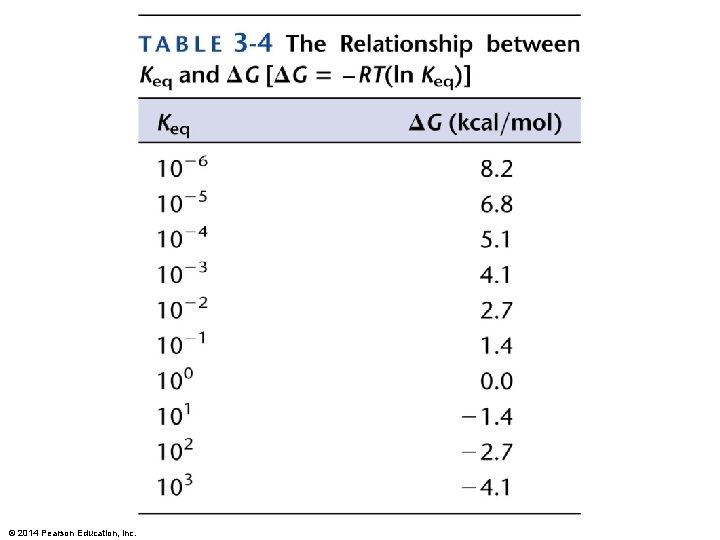
But, in biological systems, standard conditions do not exist, and DG = DG 0 + R x T x (ln [C][D]/[A][B] ) What can this tell us about why non-spontaneous reactions in a biochemical pathway can occur even without the in put of energy (sometimes)? Discuss, with your small group the following equation : DG = DH – TDS. What are the variables and what is their relevance to chemical reactions? Which of the following scenarios would not result in a decrease in free energy? A. Enthalpy increases and entropy decreases. B. Enthalpy decreases and entropy increases. C. Enthalpy increases, but entropy increase outweighs increase in enthalpy. D. Enthalpy decreases enough to outweigh a slight decrease in entropy. © 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

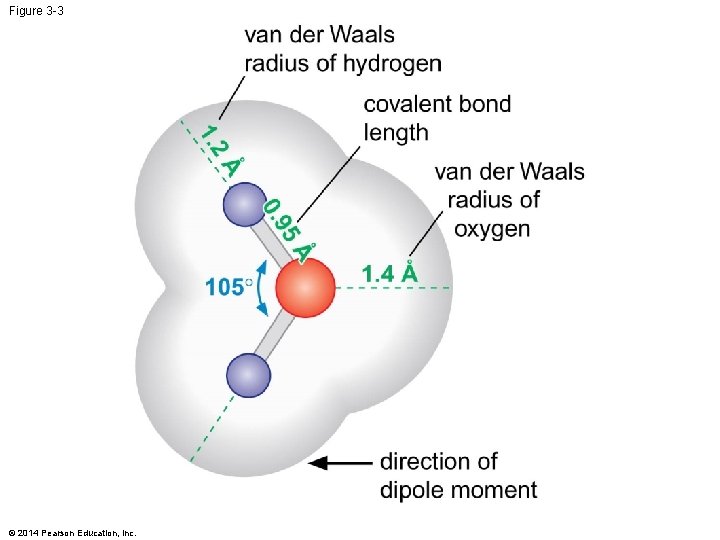
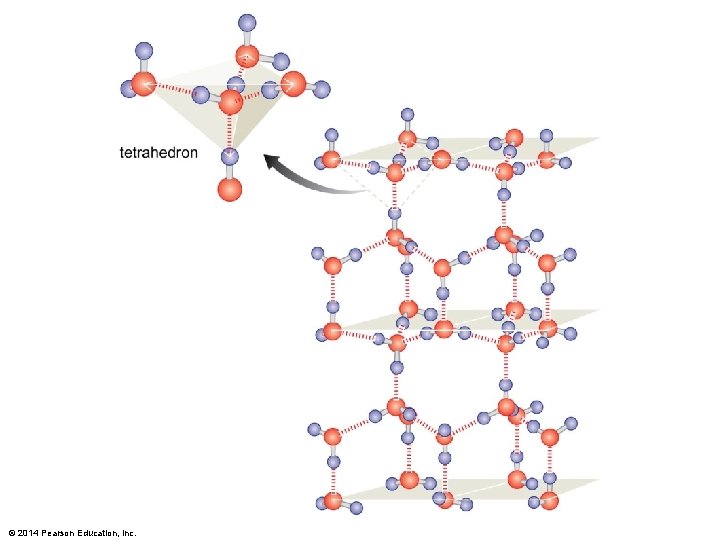
Figure 3 -3 © 2014 Pearson Education, Inc.

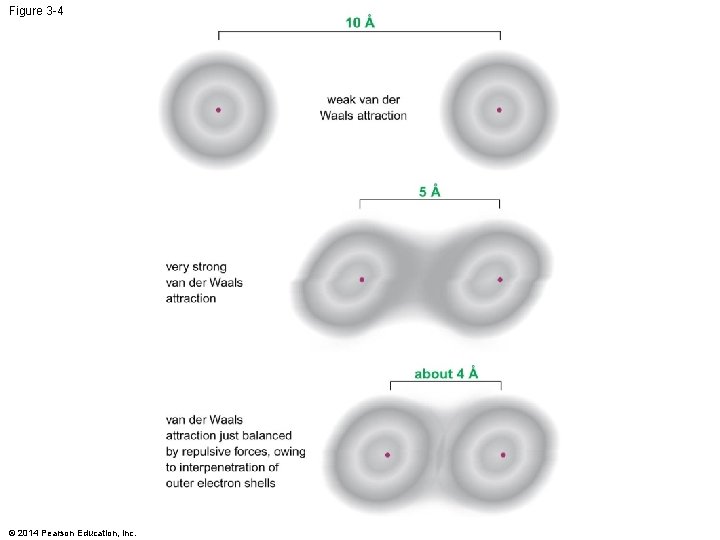
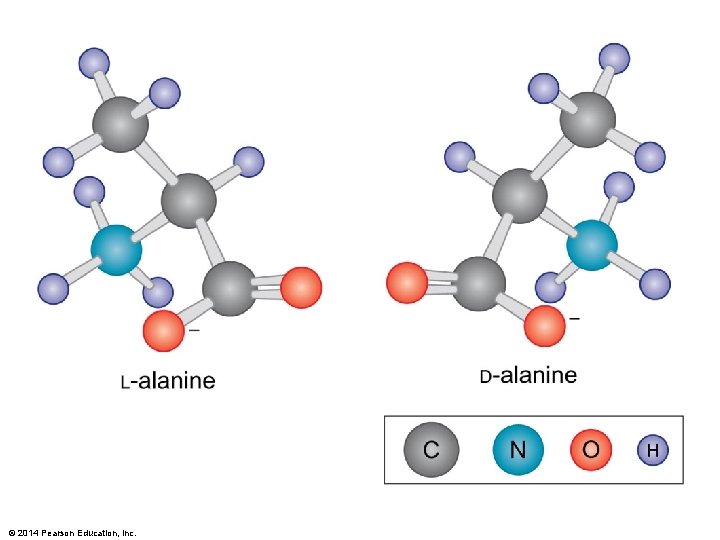
Figure 3 -4 © 2014 Pearson Education, Inc.

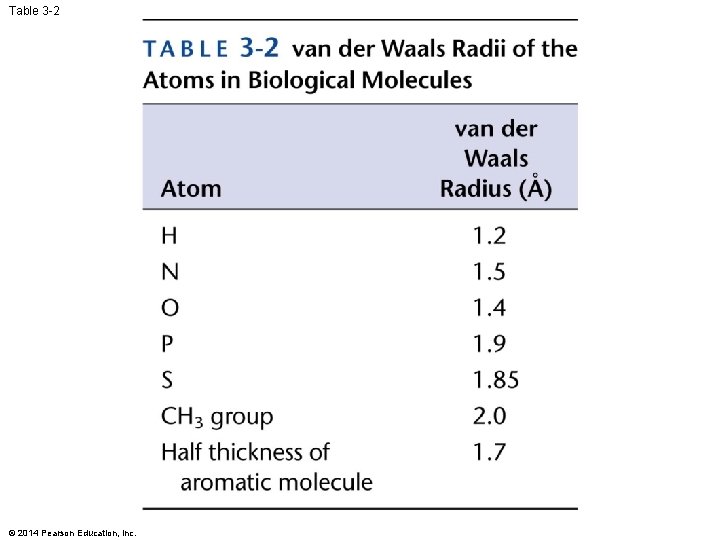
Table 3 -2 © 2014 Pearson Education, Inc.

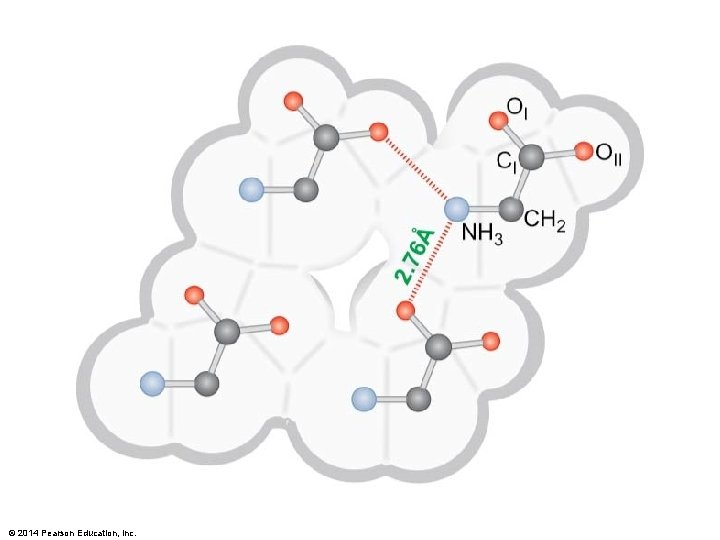
© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

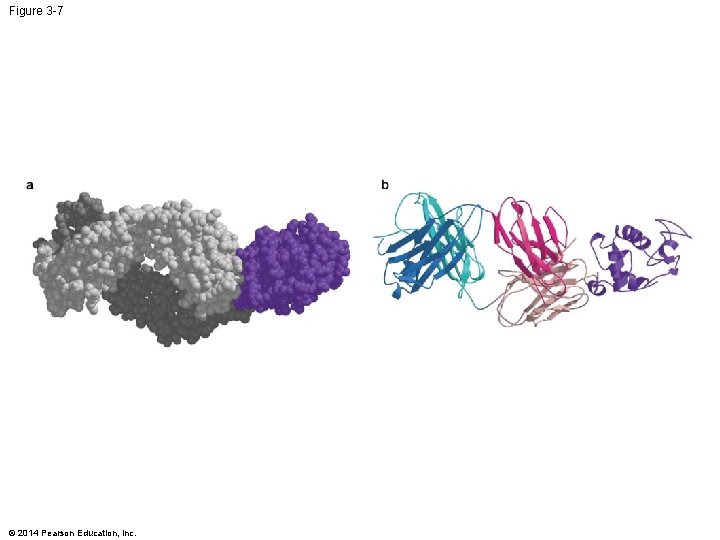
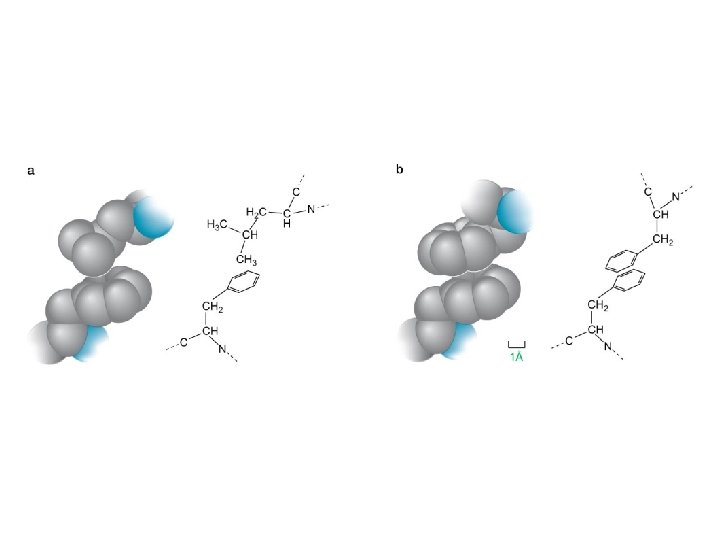
Figure 3 -7 © 2014 Pearson Education, Inc.

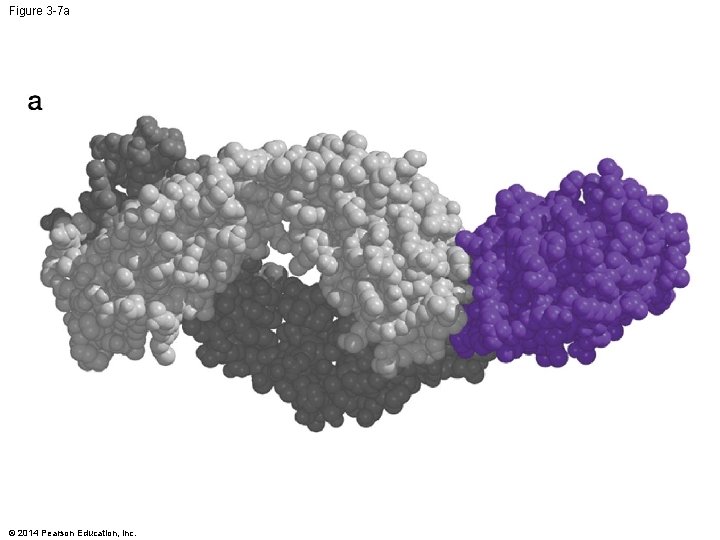
Figure 3 -7 a © 2014 Pearson Education, Inc.

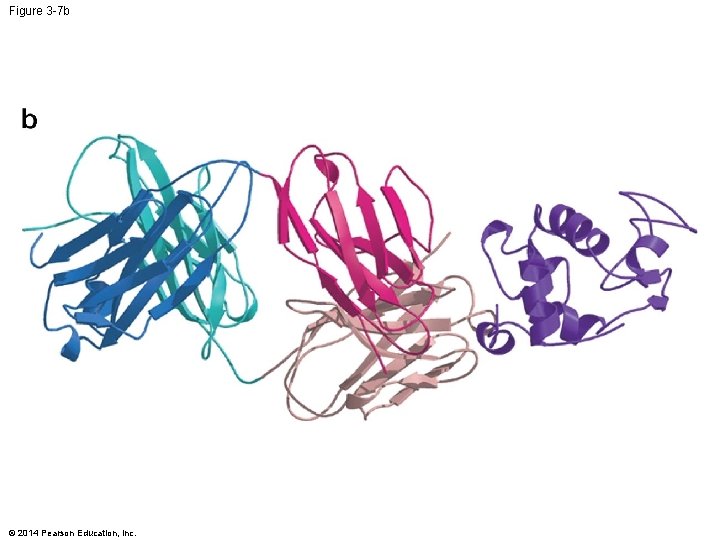
Figure 3 -7 b © 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

Figure 3 -12 © 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

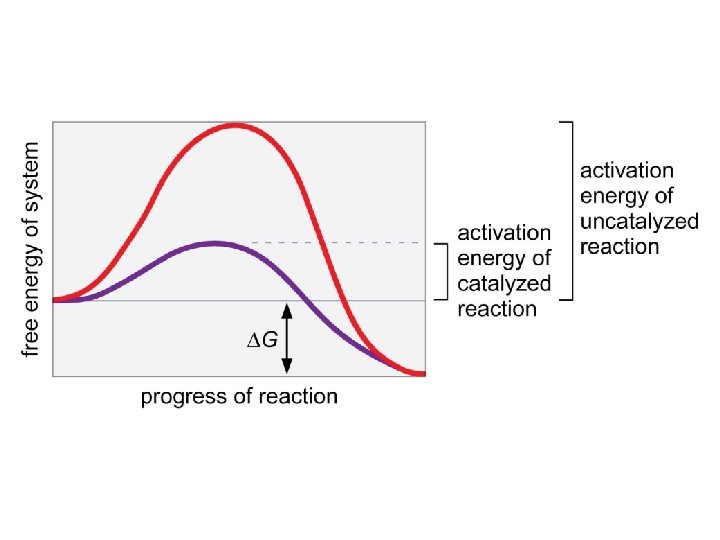
Figure 3 -13 © 2014 Pearson Education, Inc.

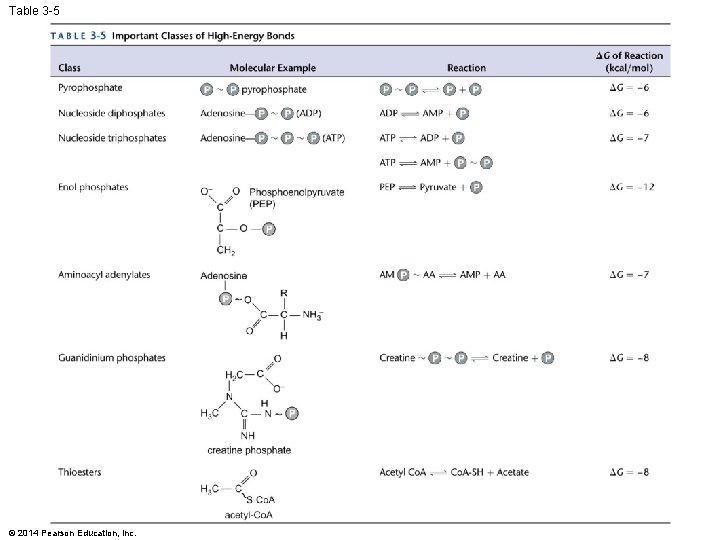
Table 3 -5 © 2014 Pearson Education, Inc.

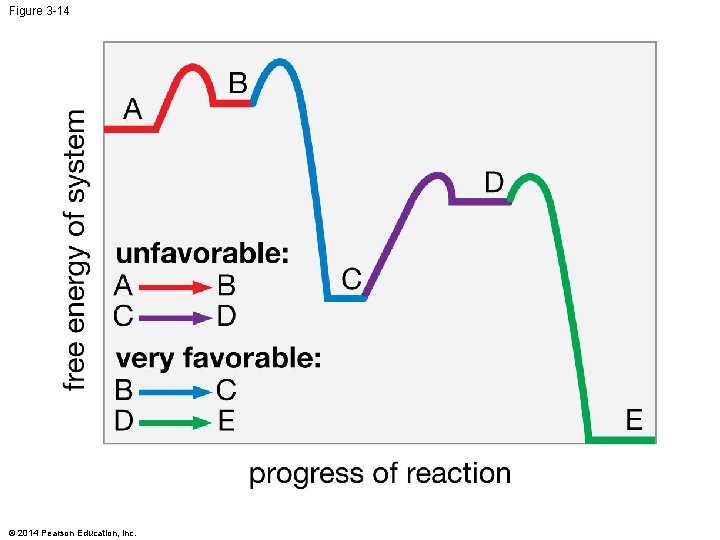
Figure 3 -14 © 2014 Pearson Education, Inc.

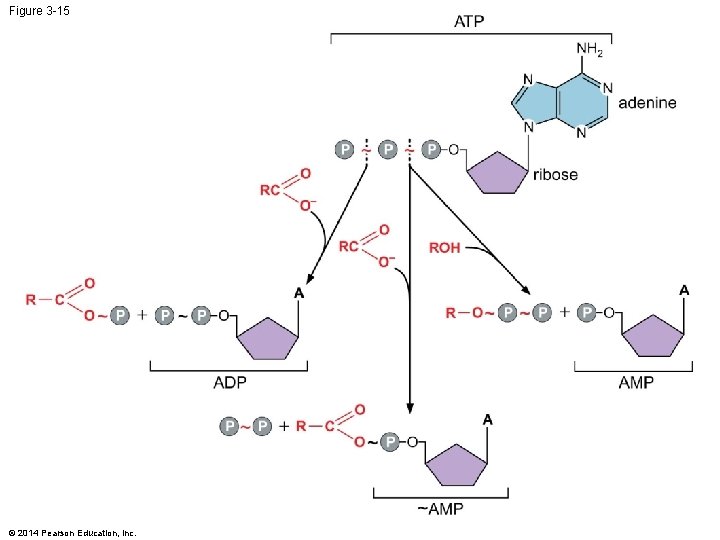
Figure 3 -15 © 2014 Pearson Education, Inc.

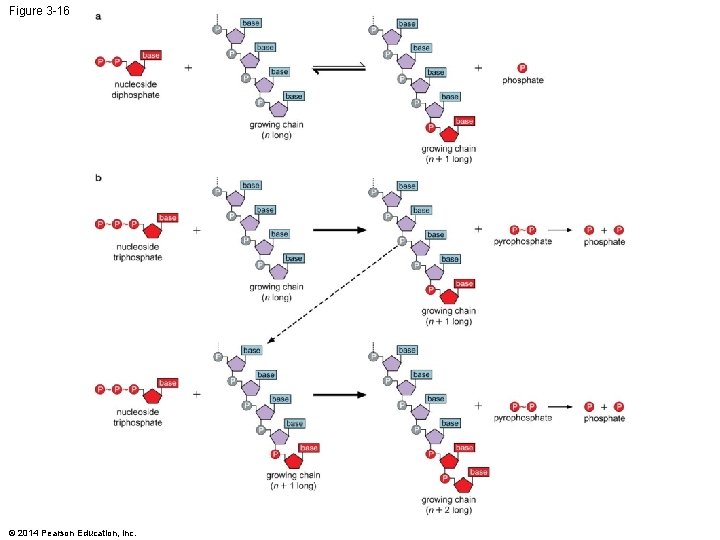
Figure 3 -16 © 2014 Pearson Education, Inc.