2015 Pearson Education Inc 2015 Pearson Education Inc





















- Slides: 38

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.
© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.
© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

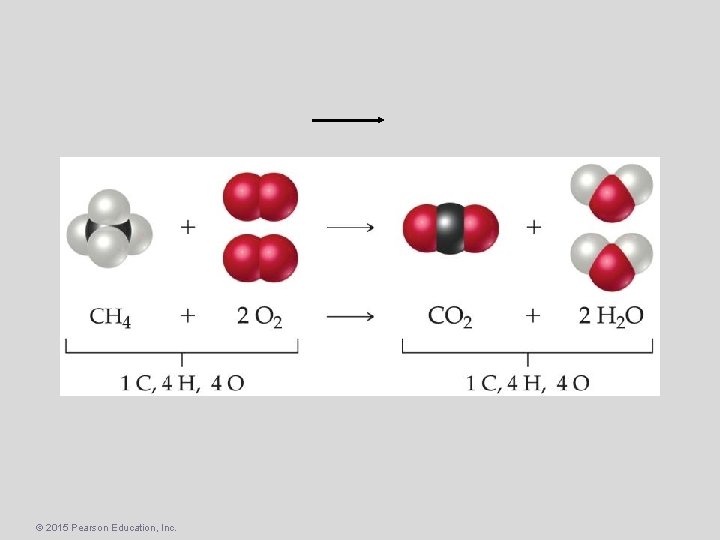
One can find the percentage of the mass of a compound that comes from each of the elements in the compound by using this equation: So the percentage of carbon in ethane is © 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

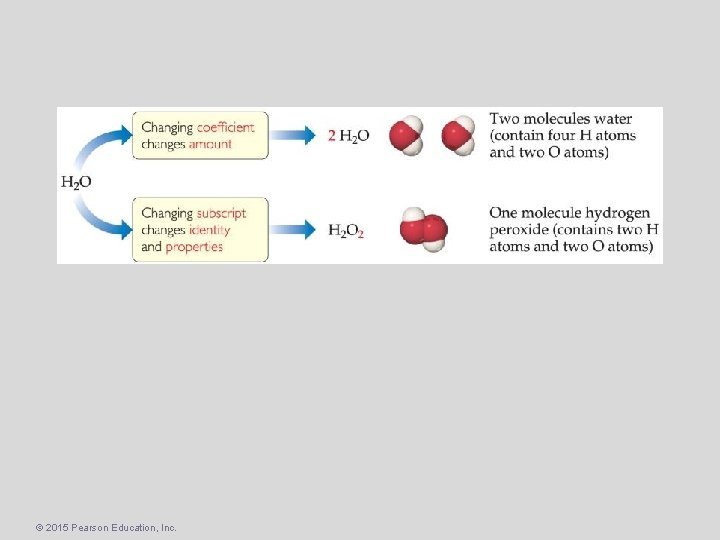
• The number of atoms in a molecular formula is a multiple of the number of atoms in an empirical formula. • If we find the empirical formula and know a molar mass (molecular weight) for the compound, we can find the molecular formula. © 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.
© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.
 2015 pearson education inc
2015 pearson education inc 2015 pearson education inc
2015 pearson education inc 2015 pearson education inc
2015 pearson education inc 2015 pearson education inc
2015 pearson education inc 2015 pearson education inc
2015 pearson education inc 2015 pearson education inc
2015 pearson education inc 2015 pearson education inc
2015 pearson education inc 2015 pearson education inc
2015 pearson education inc Pearson education inc publishing
Pearson education inc publishing 2015 pearson education inc
2015 pearson education inc Pearson education inc publishing as pearson prentice hall
Pearson education inc publishing as pearson prentice hall Pearson education inc publishing as pearson prentice hall
Pearson education inc publishing as pearson prentice hall Pearson education inc publishing as pearson prentice hall
Pearson education inc publishing as pearson prentice hall Pearson education inc publishing as pearson prentice hall
Pearson education inc publishing as pearson prentice hall 2008 pearson prentice hall inc
2008 pearson prentice hall inc Pearson education limited 2015
Pearson education limited 2015 Pearson education limited 2015
Pearson education limited 2015 Pearson education limited 2015
Pearson education limited 2015 Pearson education limited 2008
Pearson education limited 2008 Pearson education limited 2015
Pearson education limited 2015 Pearson education limited 2015
Pearson education limited 2015 Pearson education limited 2015
Pearson education limited 2015 Pearson education limited 2015
Pearson education limited 2015 Pearson education limited 2015
Pearson education limited 2015 Copyright pearson education inc
Copyright pearson education inc Copyright pearson education inc
Copyright pearson education inc 2017 pearson education inc
2017 pearson education inc 2017 pearson education inc
2017 pearson education inc 2017 pearson education inc
2017 pearson education inc 2017 pearson education inc
2017 pearson education inc 2017 pearson education inc
2017 pearson education inc 2016 pearson education inc
2016 pearson education inc 2014 pearson education inc
2014 pearson education inc 2013 pearson education inc
2013 pearson education inc 2013 pearson education inc
2013 pearson education inc 2013 pearson education inc. answers
2013 pearson education inc. answers 2013 pearson education inc
2013 pearson education inc Pearson education, inc. publishing as prentice hall
Pearson education, inc. publishing as prentice hall 2012 pearson education inc
2012 pearson education inc