11242020 P 6 Radioactive Materials OCR 21 st



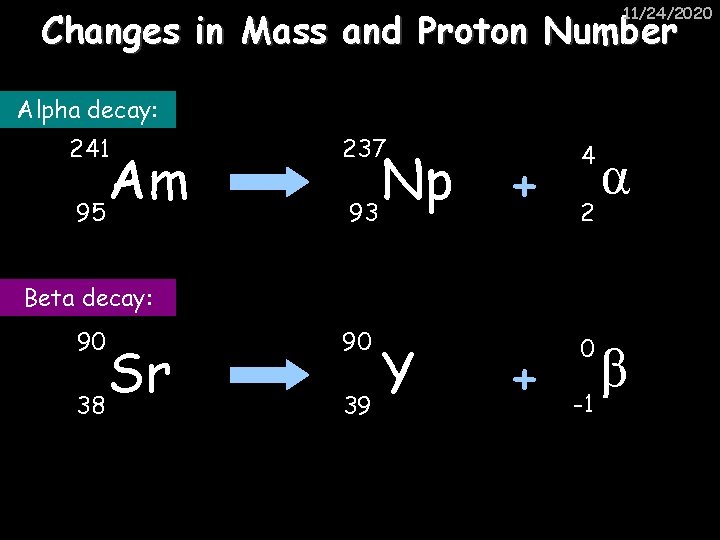





- Slides: 28

11/24/2020 P 6 Radioactive Materials OCR 21 st Century W Richards The Weald School

11/24/2020 P 6. 1 Why are some materials radioactive?

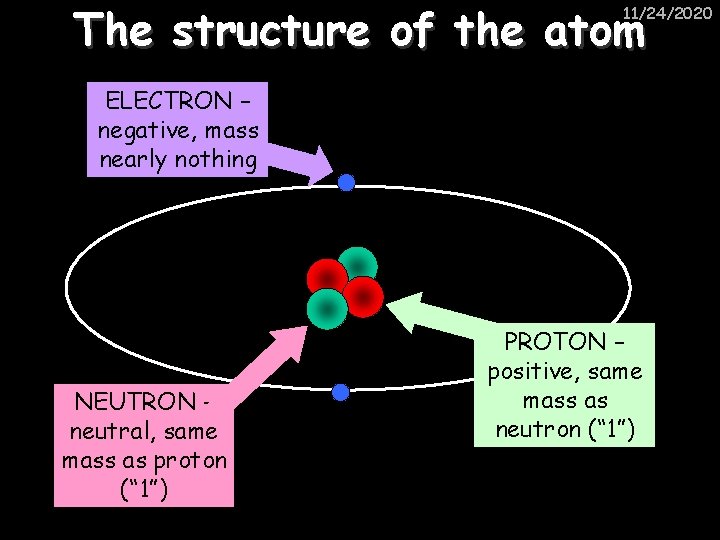
The structure of the atom 11/24/2020 ELECTRON – negative, mass nearly nothing NEUTRON – neutral, same mass as proton (“ 1”) PROTON – positive, same mass as neutron (“ 1”)

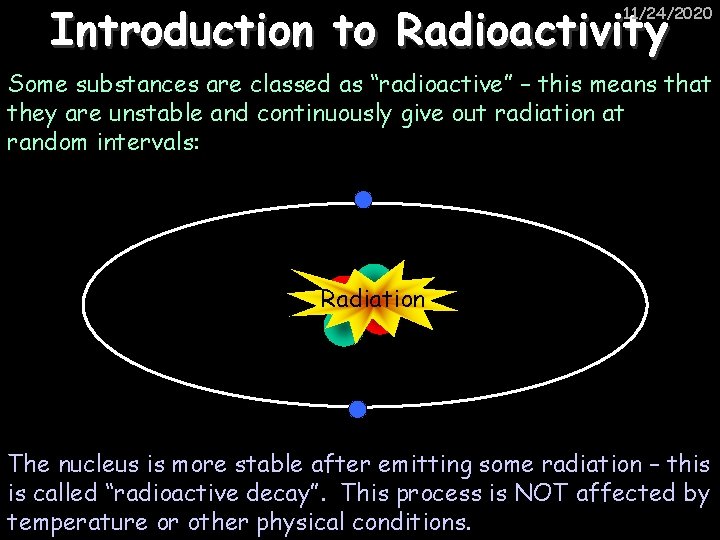
Introduction to Radioactivity 11/24/2020 Some substances are classed as “radioactive” – this means that they are unstable and continuously give out radiation at random intervals: Radiation The nucleus is more stable after emitting some radiation – this is called “radioactive decay”. This process is NOT affected by temperature or other physical conditions.

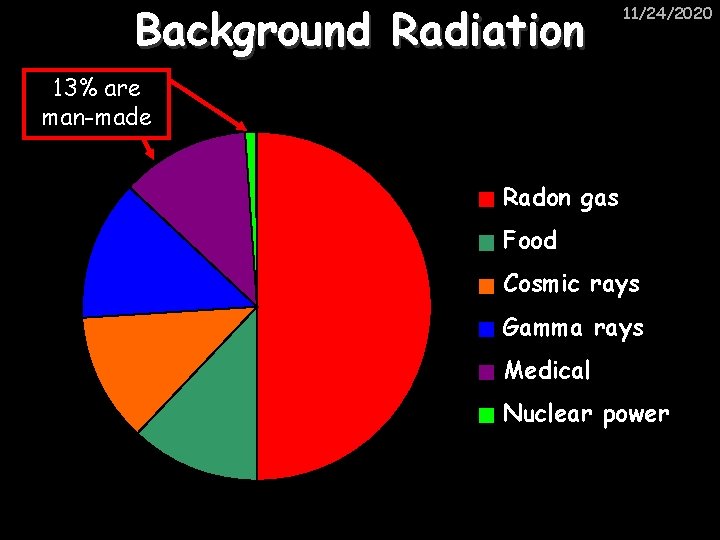
Background Radiation 11/24/2020 13% are man-made Radon gas Food Cosmic rays Gamma rays Medical Nuclear power

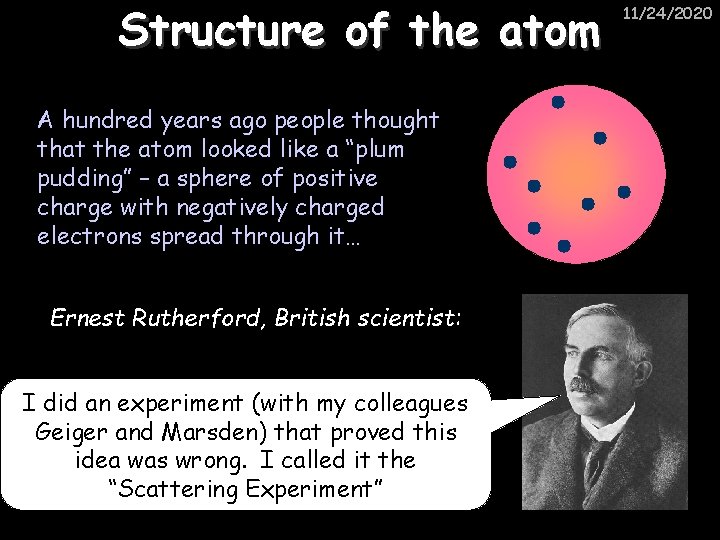
Structure of the atom A hundred years ago people thought that the atom looked like a “plum pudding” – a sphere of positive charge with negatively charged electrons spread through it… Ernest Rutherford, British scientist: I did an experiment (with my colleagues Geiger and Marsden) that proved this idea was wrong. I called it the “Scattering Experiment” 11/24/2020

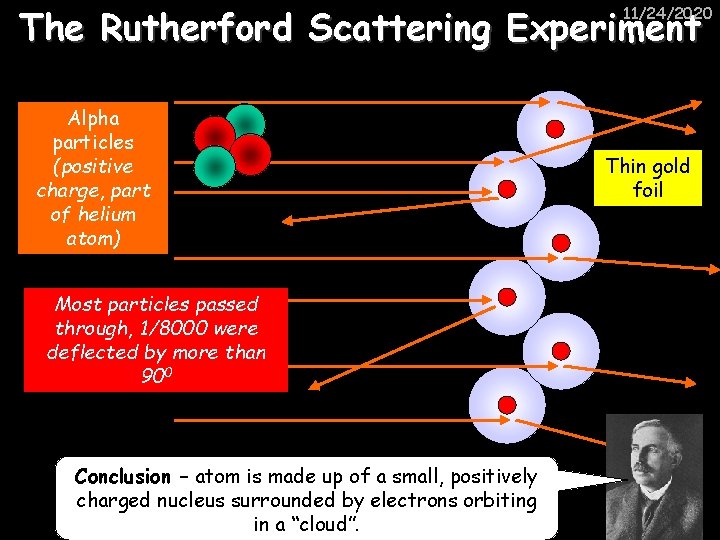
The Rutherford Scattering Experiment 11/24/2020 Alpha particles (positive charge, part of helium atom) Most particles passed through, 1/8000 were deflected by more than 900 Conclusion – atom is made up of a small, positively charged nucleus surrounded by electrons orbiting in a “cloud”. Thin gold foil

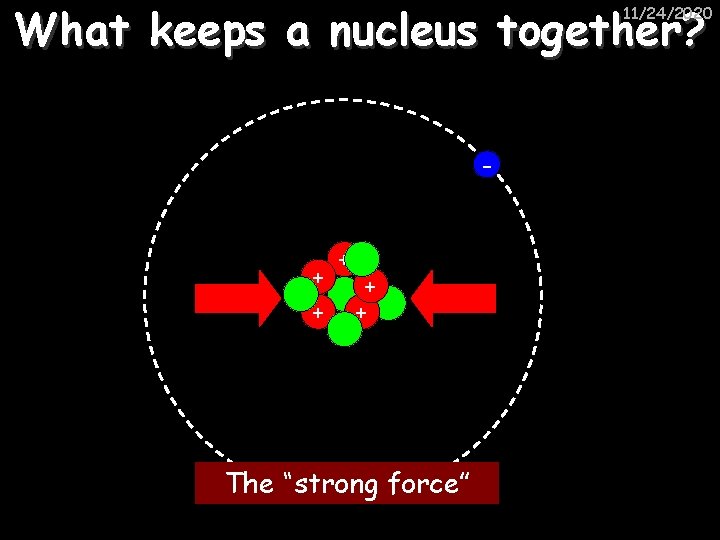
What keeps a nucleus together? 11/24/2020 - + + + The “strong force”

Nuclear Fusion in stars 11/24/2020 Nuclear Proton fusion happens in stars when hydrogen nuclei are Neutron brought close enough together: We can calculate how much energy this reaction releases using my famous E=mc 2 equation. Einstein (18791955)

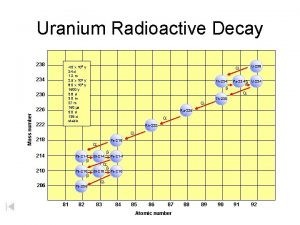
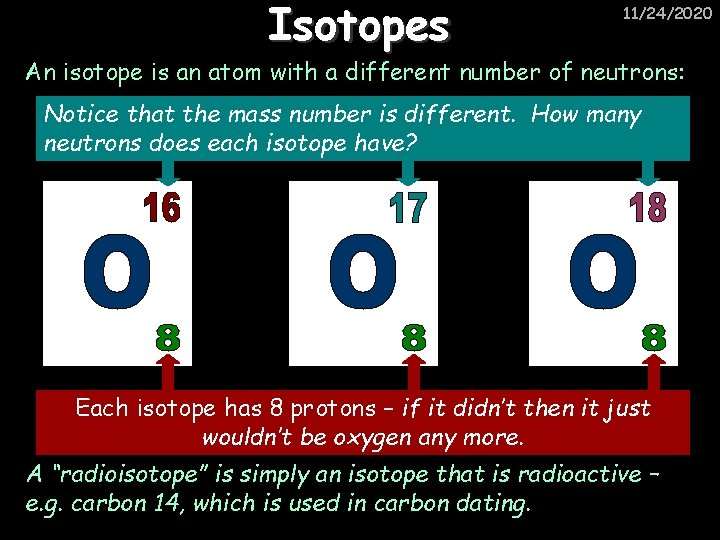
Isotopes 11/24/2020 An isotope is an atom with a different number of neutrons: Notice that the mass number is different. How many neutrons does each isotope have? Each isotope has 8 protons – if it didn’t then it just wouldn’t be oxygen any more. A “radioisotope” is simply an isotope that is radioactive – e. g. carbon 14, which is used in carbon dating.

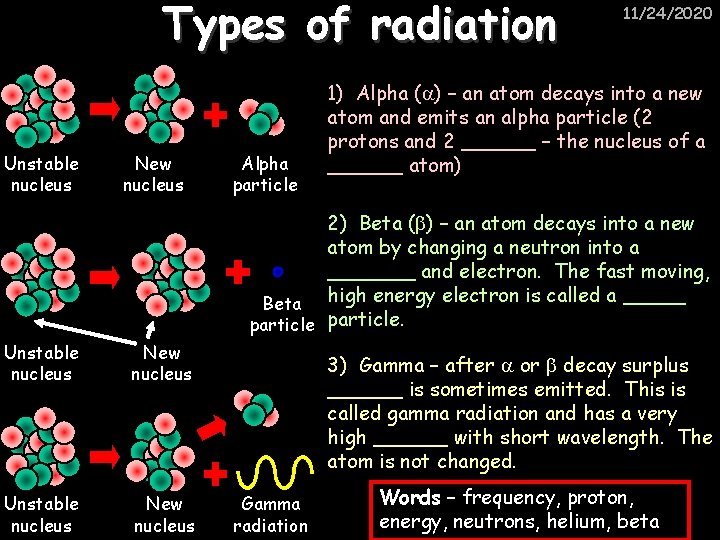
Types of radiation Unstable nucleus New nucleus Alpha particle 11/24/2020 1) Alpha ( ) – an atom decays into a new atom and emits an alpha particle (2 protons and 2 ______ – the nucleus of a ______ atom) 2) Beta ( ) – an atom decays into a new atom by changing a neutron into a _______ and electron. The fast moving, Beta high energy electron is called a _____ particle. Unstable nucleus New nucleus 3) Gamma – after or decay surplus ______ is sometimes emitted. This is called gamma radiation and has a very high ______ with short wavelength. The atom is not changed. Gamma radiation Words – frequency, proton, energy, neutrons, helium, beta

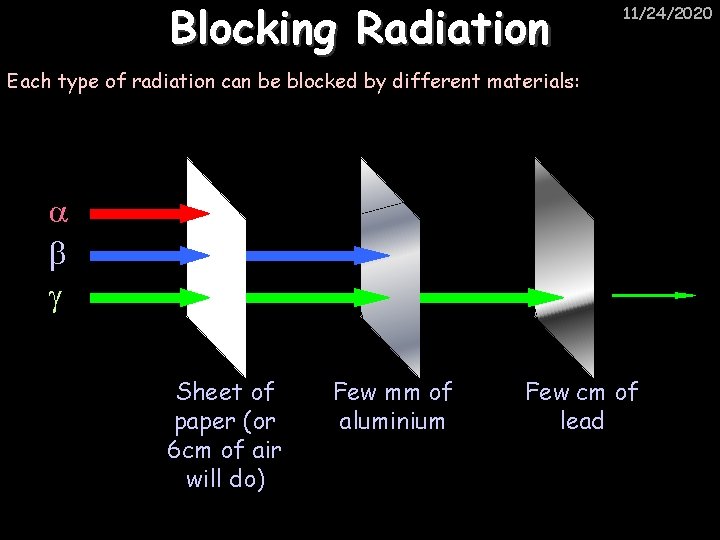
Blocking Radiation 11/24/2020 Each type of radiation can be blocked by different materials: Sheet of paper (or 6 cm of air will do) Few mm of aluminium Few cm of lead
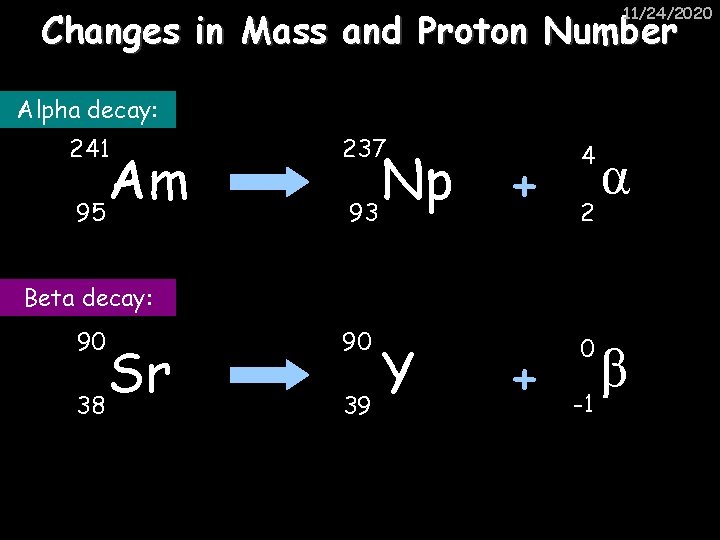
11/24/2020 Changes in Mass and Proton Number Alpha decay: 241 Am 95 237 Np 93 + 4 + 0 2 α Beta decay: 90 Sr 38 90 Y 39 β -1

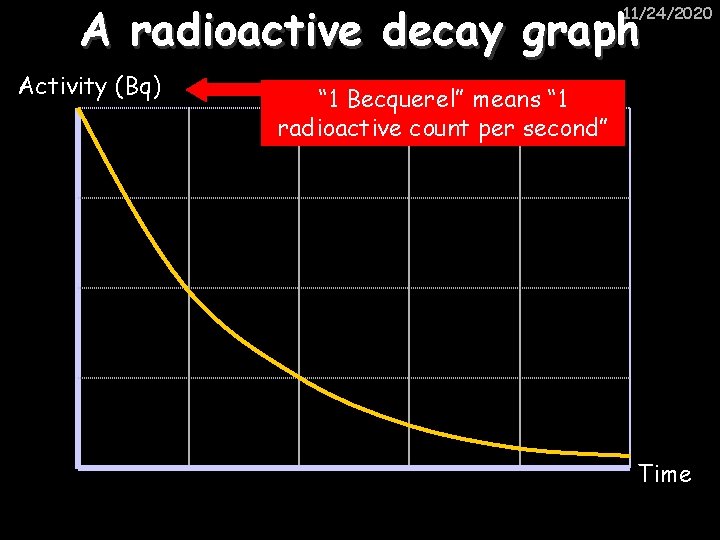
A radioactive decay graph 11/24/2020 Activity (Bq) “ 1 Becquerel” means “ 1 radioactive count per second” Time

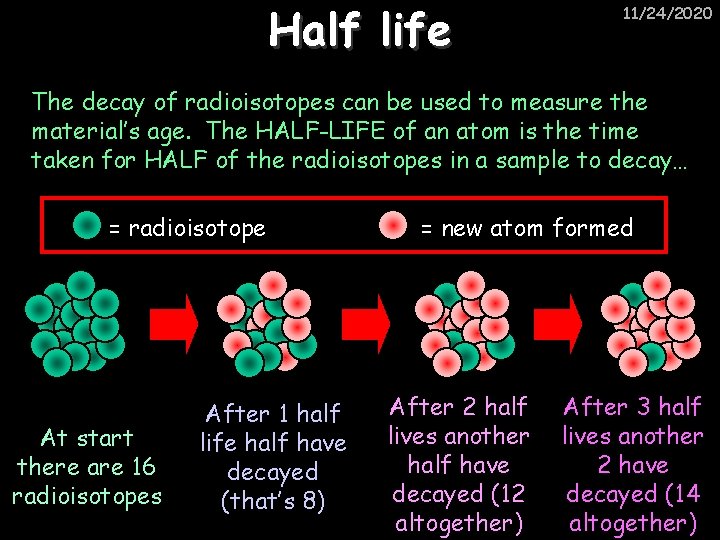
Half life 11/24/2020 The decay of radioisotopes can be used to measure the material’s age. The HALF-LIFE of an atom is the time taken for HALF of the radioisotopes in a sample to decay… = radioisotope At start there are 16 radioisotopes After 1 half life half have decayed (that’s 8) = new atom formed After 2 half lives another half have decayed (12 altogether) After 3 half lives another 2 have decayed (14 altogether)

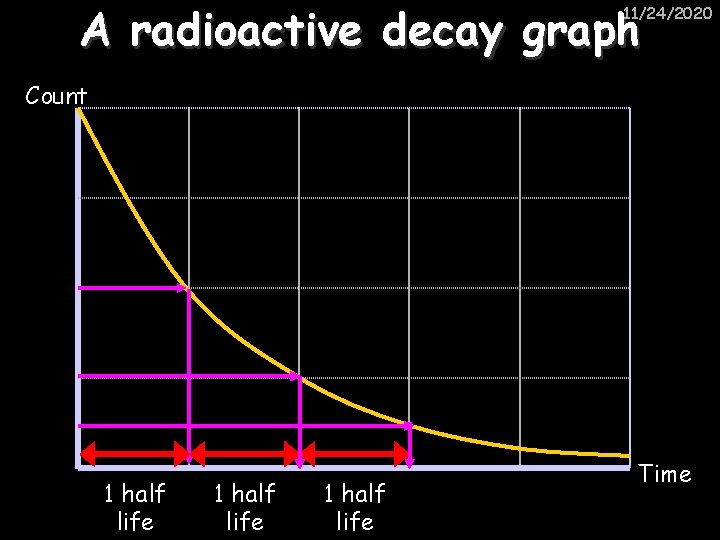
A radioactive decay graph 11/24/2020 Count 1 half life Time

P 6. 2 Using Radioactive Materials 11/24/2020

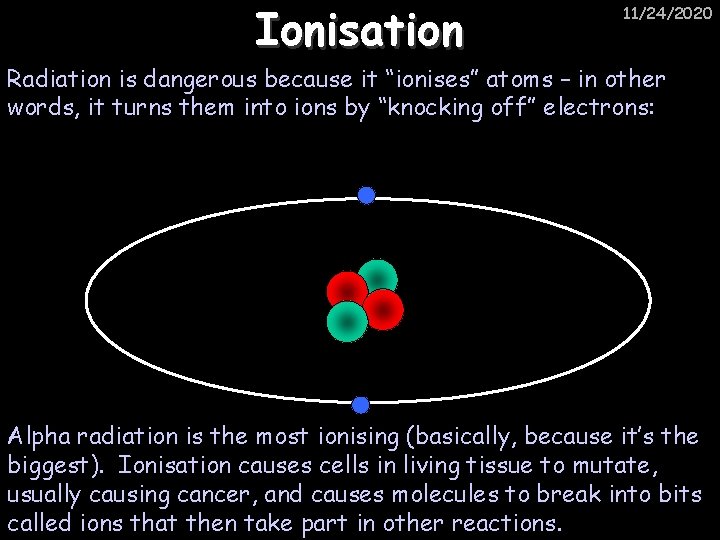
Ionisation 11/24/2020 Radiation is dangerous because it “ionises” atoms – in other words, it turns them into ions by “knocking off” electrons: Alpha radiation is the most ionising (basically, because it’s the biggest). Ionisation causes cells in living tissue to mutate, usually causing cancer, and causes molecules to break into bits called ions that then take part in other reactions.

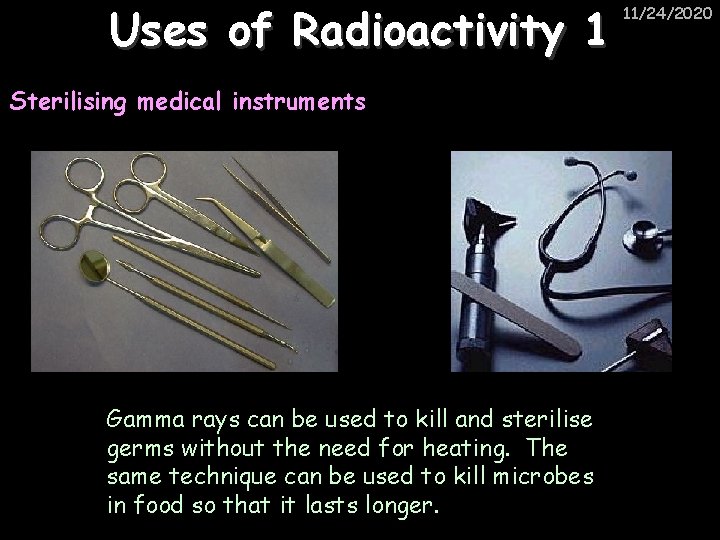
Uses of Radioactivity 1 Sterilising medical instruments Gamma rays can be used to kill and sterilise germs without the need for heating. The same technique can be used to kill microbes in food so that it lasts longer. 11/24/2020

Uses of Radioactivity 2 - Tracers 11/24/2020 A tracer is a small amount of radioactive material used to detect things, e. g. a leak in a pipe: Gamma source The radiation from the radioactive source is picked up above the ground, enabling the leak in the pipe to be detected. Tracers can also be used in medicine to detect tumours: For medicinal tracers, you would probably use a beta source with a short half life – why?

11/24/2020 Uses of Radioactivity 3 - Treating Cancer High energy gamma radiation can be used to kill cancerous cells. However, care must be taken in order to enure that the gamma radiation does not affect normal tissue as well. Radioactive iodine can be used to treat thyroid cancer. Iodine is needed by the thyroid so it naturally collects there. Radioactive iodine will then give out beta radiation and kill cancerous cells. What sort of half life would you want the radioactive iodine to have?

Exposure to Radiation 11/24/2020 People like me work with radiation a lot so we need to wear a “dosimeter” to record our exposure to radiation: Radiation dose is measured in units called “sieverts” (Sv).

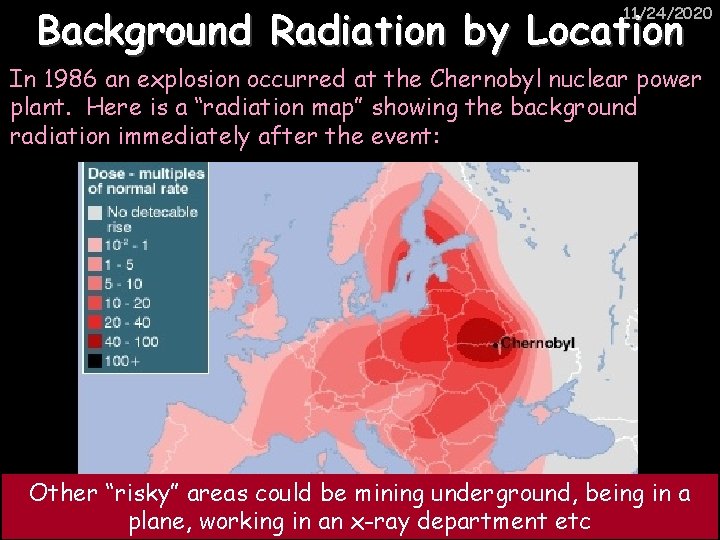
Background Radiation by Location 11/24/2020 In 1986 an explosion occurred at the Chernobyl nuclear power plant. Here is a “radiation map” showing the background radiation immediately after the event: Other “risky” areas could be mining underground, being in a plane, working in an x-ray department etc

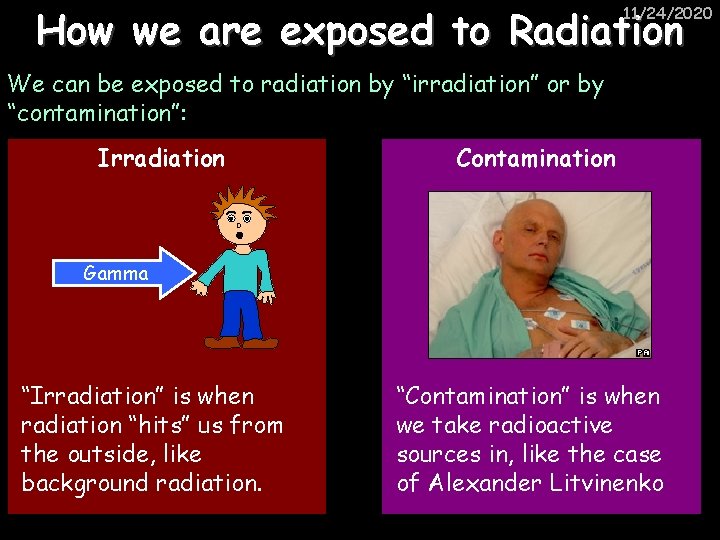
How we are exposed to Radiation 11/24/2020 We can be exposed to radiation by “irradiation” or by “contamination”: Irradiation Contamination Gamma “Irradiation” is when radiation “hits” us from the outside, like background radiation. “Contamination” is when we take radioactive sources in, like the case of Alexander Litvinenko

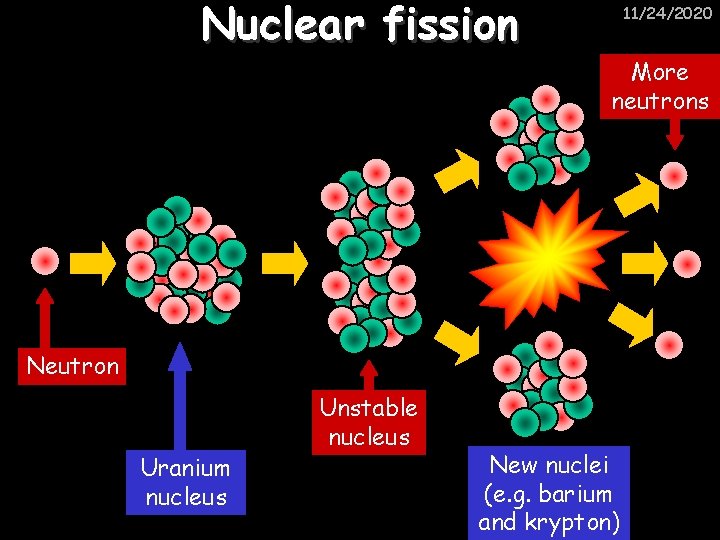
Nuclear fission 11/24/2020 More neutrons Neutron Uranium nucleus Unstable nucleus New nuclei (e. g. barium and krypton)

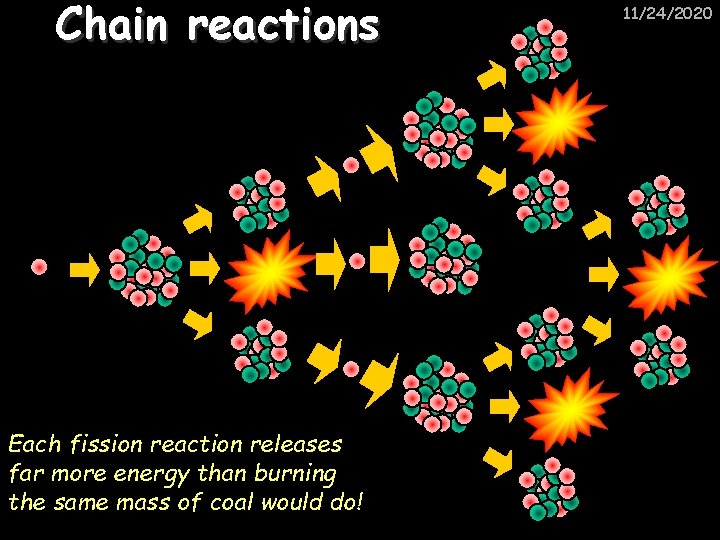
Chain reactions Each fission reaction releases far more energy than burning the same mass of coal would do! 11/24/2020

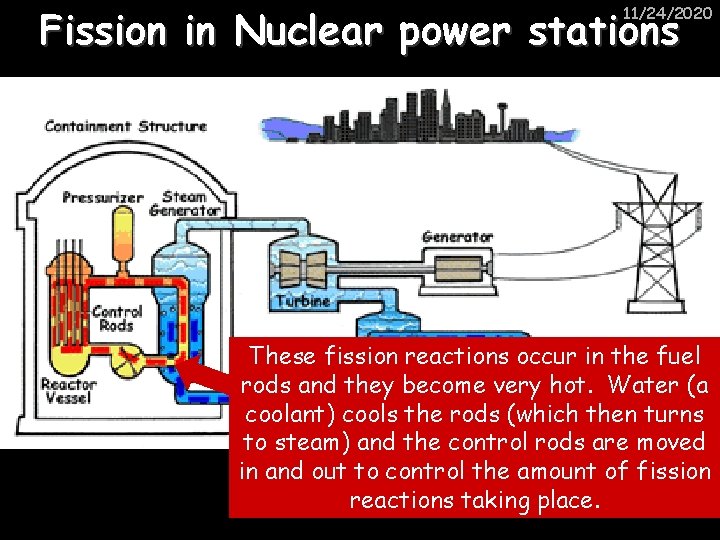
Fission in Nuclear power stations 11/24/2020 These fission reactions occur in the fuel rods and they become very hot. Water (a coolant) cools the rods (which then turns to steam) and the control rods are moved in and out to control the amount of fission reactions taking place.

Disposing of radioactive waste 11/24/2020 The key to dealing with radioactive waste is to IMMOBILISE it. There a number of ways of doing this depending on how _____ the waste is: High level waste is immobilised by mixing with ____ making ingredients, melting and pouring the glass into steel containers. Intermediate waste is set in cement in _____ drums. The containers are then kept in stores, often _____. Words – glass, steel, underground, radioactive
 Radioactive materials have unstable
Radioactive materials have unstable Radioactive materials logistics market
Radioactive materials logistics market Ocr sample assessment materials
Ocr sample assessment materials Man made map
Man made map What is adopting materials
What is adopting materials Go noodle cant stop the feeling
Go noodle cant stop the feeling Direct materials budget with multiple materials
Direct materials budget with multiple materials Harmful or useful
Harmful or useful Chapter 24: nuclear chemistry answer key
Chapter 24: nuclear chemistry answer key Chemistry
Chemistry Discrete radioactive particles
Discrete radioactive particles Radioactive nuclear waste
Radioactive nuclear waste Discovery of nucleus of atom
Discovery of nucleus of atom Radiation pollution conclusion
Radiation pollution conclusion Radioactive
Radioactive Radioactive waste
Radioactive waste Radioactive fallout from chernobyl
Radioactive fallout from chernobyl Uses of radioactive isotopes
Uses of radioactive isotopes Radioactive decay formula
Radioactive decay formula Gamma emission equation
Gamma emission equation Radioactive defination
Radioactive defination Radioactive flac
Radioactive flac Most unstable radioactive element
Most unstable radioactive element Gamma decay nuclear equation
Gamma decay nuclear equation Is lead radioactive
Is lead radioactive Radioactive rays
Radioactive rays Radioactive tracers in agriculture
Radioactive tracers in agriculture Law of radioactive decay
Law of radioactive decay Calculation of radioactive decay
Calculation of radioactive decay