Water Treatment Sedimentation Filtration and Disinfection SedimentationSettling Following














- Slides: 26

Water Treatment: Sedimentation, Filtration and Disinfection

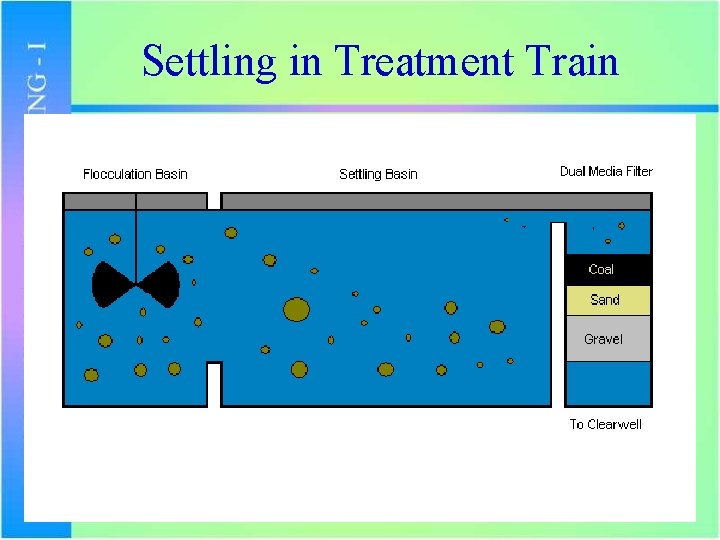
Sedimentation/Settling • Following flocculation, the water then flows into the settling basins • Water is nearly inactive – low flow with little turbulence • Water resides for at least 3 hours and the flocs settle out and collect at the bottom.

Settling in Treatment Train

Circular Clarifiers

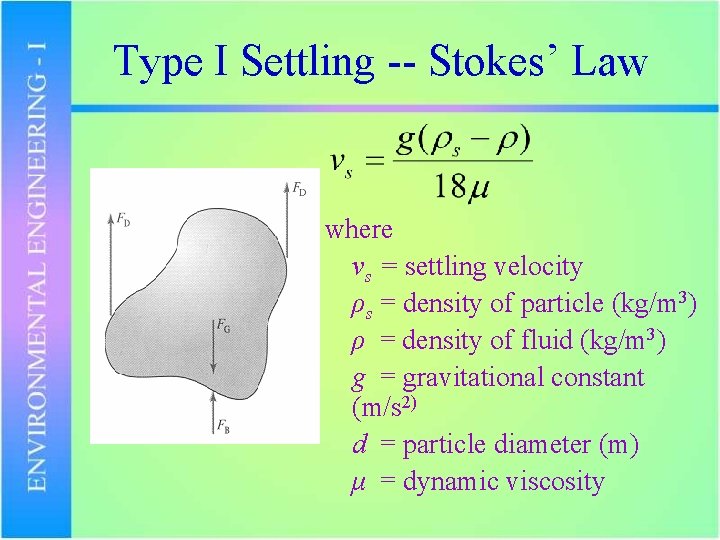
Type I Settling -- Stokes’ Law where νs = settling velocity ρs = density of particle (kg/m 3) ρ = density of fluid (kg/m 3) g = gravitational constant (m/s 2) d = particle diameter (m) μ = dynamic viscosity

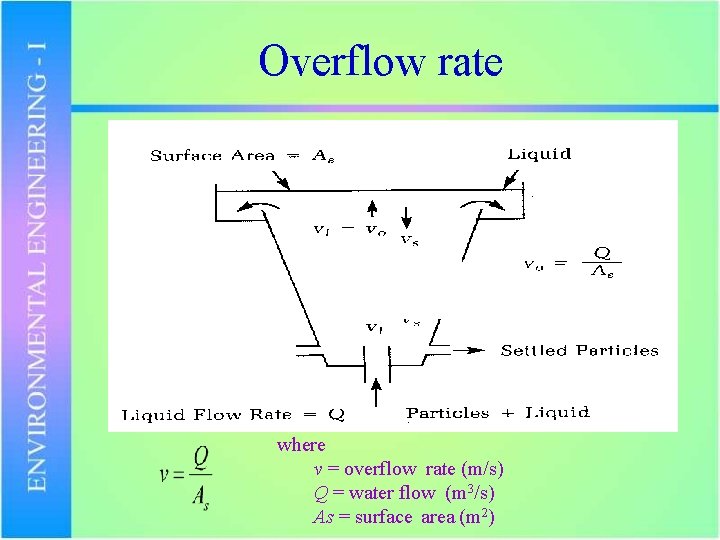
Overflow rate where v = overflow rate (m/s) Q = water flow (m 3/s) As = surface area (m 2)

Types of Particle Settling • Type I settling applies to particles that settle with constant velocity -- particles will be removed if v > vs • Type II settling if particles flocculate during settling, velocity generally increases • Type III As particle concentration increases with depth, zone settling occurs • Type IV At bottom of tank compression settling occurs

Filtration • The final step in removing particles is filtration. • Removal of those particles that are too small to be effectively removed during sedimentation • Multiple removal mechanisms depending on design

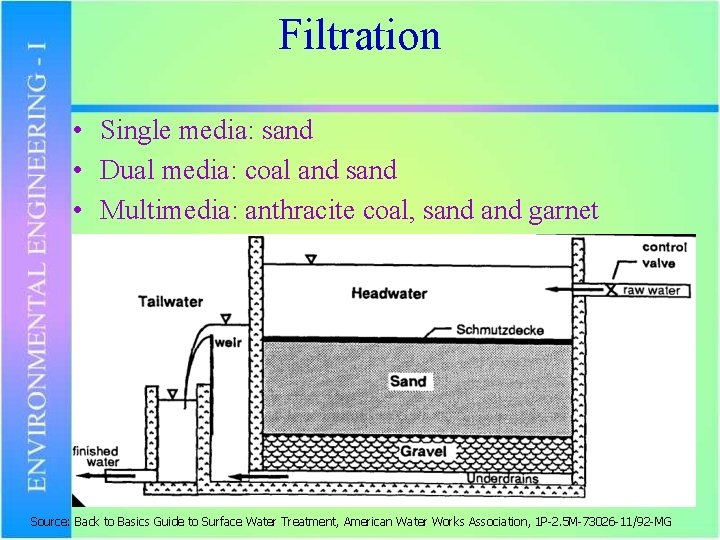
Filtration • Single media: sand • Dual media: coal and sand • Multimedia: anthracite coal, sand garnet Source: Back to Basics Guide to Surface Water Treatment, American Water Works Association, 1 P-2. 5 M-73026 -11/92 -MG

Filtration

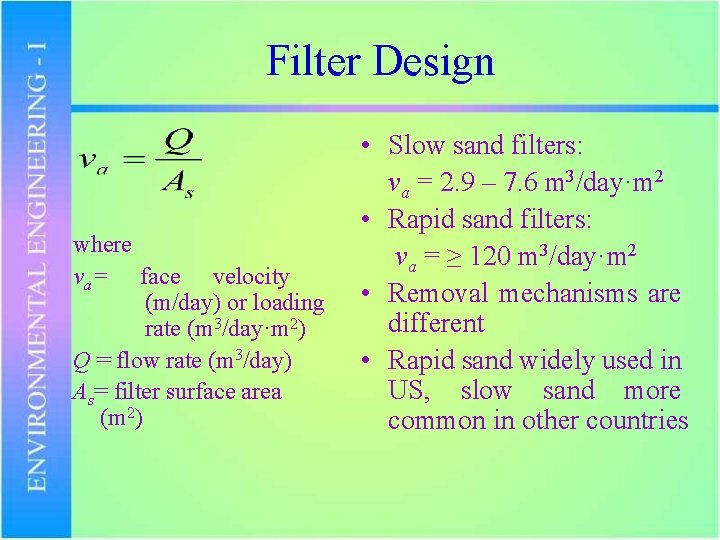
Filter Design where va= face velocity (m/day) or loading rate (m 3/day·m 2) Q = flow rate (m 3/day) As= filter surface area (m 2) • Slow sand filters: va = 2. 9 – 7. 6 m 3/day·m 2 • Rapid sand filters: va = ≥ 120 m 3/day·m 2 • Removal mechanisms are different • Rapid sand widely used in US, slow sand more common in other countries

Rapid Sand Filtration • As particles are removed - filter becomes clogged – head loss increases, turbidity increases • Must backwash (takes about 10 -15 min) done about once per day • Must design to handle flow with one filter out of service

Rapid Sand Filtration • Backwashing is accomplished by forcing water (and sometimes air) up from the clearwell back through the filter. • The particles in the filter become suspended, releasing the trapped particles. • Backwash water retreated or disposed of.

Disinfection • Following filtration, water is disinfected • Chlorine gas is most commonly used • Two design goals – kill majority of organisms in water – provide residual disinfection capability to prevent growth of organisms in distribution system

Chlorine Reactions in Water • Cl 2 (g) + H 2 O = HOCl + H+ + Cl– p. H dependent – essentially complete within a few milliseconds • HOCl = H+ + OCl– HOCl is about 80 - 100 times more effective than is OCl- for E. Coli – [HOCl] + [OCl-] = free available chlorine • HOCl + NH 3 = NH 2 Cl + H 2 O – NH 2 Cl (monochloramine) is less effective but longer lasting – combined chlorine

Other Disinfectants • Hypochlorite salts: Na. OCl and Ca(OCl)2 – more expensive to purchase – easier to handle – more common for small supplies • Chloramines (NH 2 Cl, NHCl 2, NCl 3) – longer contact time if primary disinfectant – used in combination with other disinfectants • Chlorine dioxide (Cl. O 2) – very effective – must be produced on site

Other Disinfectants • Ozone (O 3) – – – very powerful oxidant no taste and odor problems widely used in Europe no residual more expensive than chlorine (produced on-site) • Ultraviolet radiation – effective bactericide and viricide – water must be free of turbidity and lamps free of slime and precipitates – no residual protection

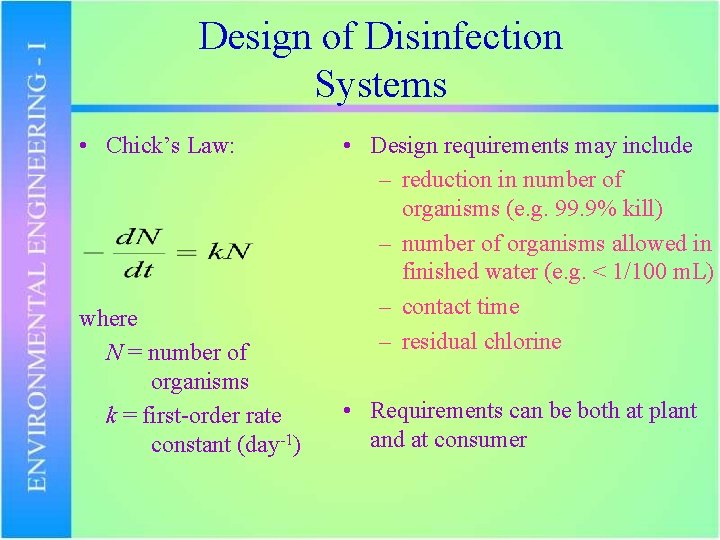
Design of Disinfection Systems • Chick’s Law: where N = number of organisms k = first-order rate constant (day-1) • Design requirements may include – reduction in number of organisms (e. g. 99. 9% kill) – number of organisms allowed in finished water (e. g. < 1/100 m. L) – contact time – residual chlorine • Requirements can be both at plant and at consumer

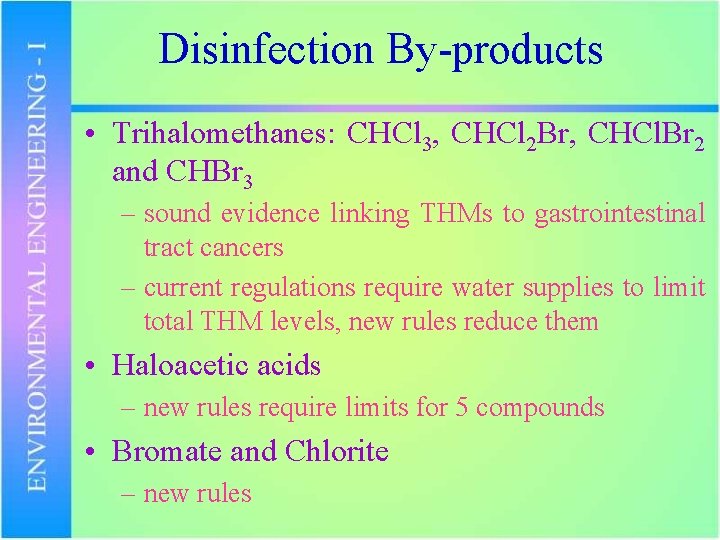
Disinfection By-products • Trihalomethanes: CHCl 3, CHCl 2 Br, CHCl. Br 2 and CHBr 3 – sound evidence linking THMs to gastrointestinal tract cancers – current regulations require water supplies to limit total THM levels, new rules reduce them • Haloacetic acids – new rules require limits for 5 compounds • Bromate and Chlorite – new rules

Additional Processes - p. H Adjustment • Recarbonation for softened water CO 2 + H 2 O H 2 CO 3 – Purpose is to reduce p. H following softening (p. H > 11 required for Mg removal) • Sodium hydroxide addition for surface water – coagulant chemicals reduce p. H – increase p. H to reduce corrosivity

Additional Processes • Polyphosphate addition – Added for corrosion control as it forms a protective film on pipes – Also helps to control lead levels in tap water as it complexes with lead

Additional Processes • Fluoride addition – Added either as Na. F, Na 2 Si. F 6, H 2 Si. F 6 – React in water to yield fluoride ion (F-) • Well documented that fluoride levels of ~ 1 ppm reduce incidence of dental caries (cavities) • Some controversy remains

Advanced Treatment Processes • Advanced Oxidation Processes – improved disinfection – oxidize synthetic organic chemicals – taste and odor control • Activated carbon adsorption – remove recalcitrant synthetic organic chemicals, THMs, taste and odor compounds – concern with bacterial growth problems • Membrane process – discriminate on both size and chemistry – selective removal including desalinzation

Residuals Management Sludge from clarifiers Finished water

Residuals Management – Dewatering • • Lagoons Sand-dying beds Freeze treatment Centrifugation Vacuum filtration Continuous belt filter press Plate Pressure filters

Residuals Management – Ultimate Disposal • • • On-site storage Land-filling Land application – soil amendment Reclamation/recycling – new products Ocean dumping – banned in most of the countries trough out the world
 Water and water and water water
Water and water and water water Cartridge filter design calculation
Cartridge filter design calculation Purification of water on large scale storage
Purification of water on large scale storage Pictures of mixtures
Pictures of mixtures Sedimentation and flotation
Sedimentation and flotation Low level disinfectant
Low level disinfectant Sterilization and disinfection
Sterilization and disinfection Sterilization and disinfection
Sterilization and disinfection Sterilization and disinfection
Sterilization and disinfection Introduction of disinfection
Introduction of disinfection Nurses responsibility in sterilization
Nurses responsibility in sterilization Sterilization and disinfection
Sterilization and disinfection Disinfection and sterilization
Disinfection and sterilization Horrocks apparatus
Horrocks apparatus Sterilization and disinfection in microbiology lab
Sterilization and disinfection in microbiology lab How to make a water filtration system for a school project
How to make a water filtration system for a school project Importance of sieving
Importance of sieving Sedimentation
Sedimentation Sedimentation in downstream processing
Sedimentation in downstream processing Corrected esr formula
Corrected esr formula Concentration techniques in parasitology
Concentration techniques in parasitology Loi de stockes
Loi de stockes Erythocyte sedimentation rate
Erythocyte sedimentation rate Sedimentation technique for stool concentration
Sedimentation technique for stool concentration Cycle de sédimentation
Cycle de sédimentation Sedimentation technique for stool concentration
Sedimentation technique for stool concentration Ova cysts and parasites
Ova cysts and parasites