WATER Ira Waluyo Nilsson Group Stanford Synchrotron Radiation





- Slides: 18

WATER Ira Waluyo Nilsson Group Stanford Synchrotron Radiation Lightsource SASS Talk 10/14/09

Ban DHMO! www. dhmo. org “DHMO is a colorless and odorless chemical compound…Its basis is the highly reactive hydroxyl radical, a species shown to mutate DNA, denature proteins, disrupt cell membranes, and chemically alter critical neurotransmitters. ” Some dangers of DHMO • Death due to accidental inhalation of DHMO, even in small quantities. • Prolonged exposure to solid DHMO causes severe tissue damage. • Gaseous DHMO can cause severe burns. • Contributes to soil erosion. • Often associated with killer cyclones in the U. S. Midwest and elsewhere, and in hurricanes including deadly storms in Florida, New Orleans and other areas of the southeastern U. S

What is DHMO? “Dihydrogen monoxide” H 2 O a. k. a water It is everywhere Covers 2/3 of Earth’s surface Comprises 50 -65% of human body No water = no life But it exhibits strange properties and it’s liquid structure is still a mystery….

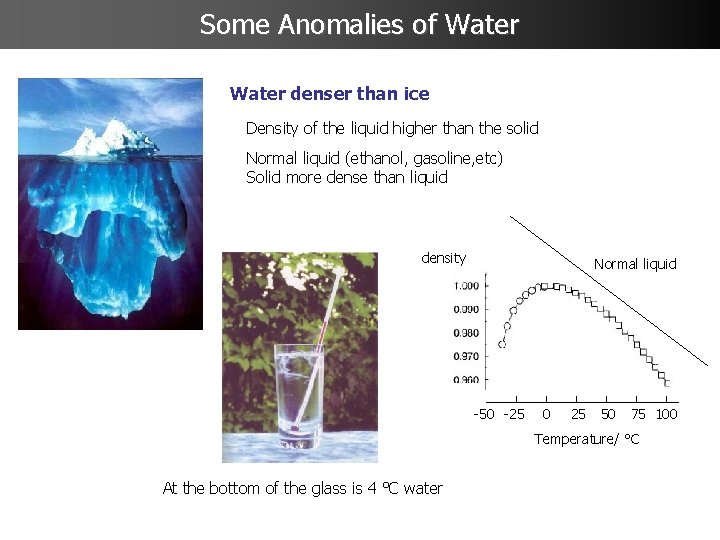
Some Anomalies of Water denser than ice Density of the liquid higher than the solid Normal liquid (ethanol, gasoline, etc) Solid more dense than liquid density Normal liquid ddd ddd Ssssssssssss -50 -25 0 25 50 75 100 s Temperature/ °C At the bottom of the glass is 4 °C water

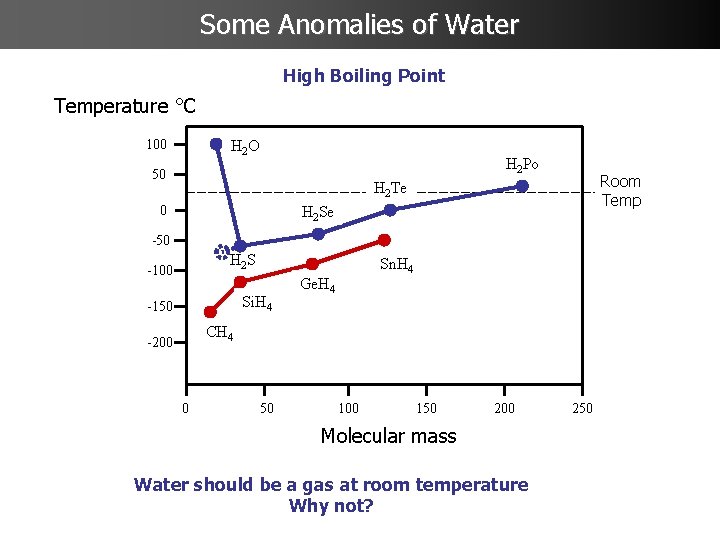
Some Anomalies of Water High Boiling Point Temperature °C 100 H 2 O H 2 Po 50 Room Temp H 2 Te 0 H 2 Se -50 H 2 S -100 Si. H 4 -150 Sn. H 4 Ge. H 4 CH 4 -200 0 50 100 150 200 Molecular mass Water should be a gas at room temperature Why not? 250

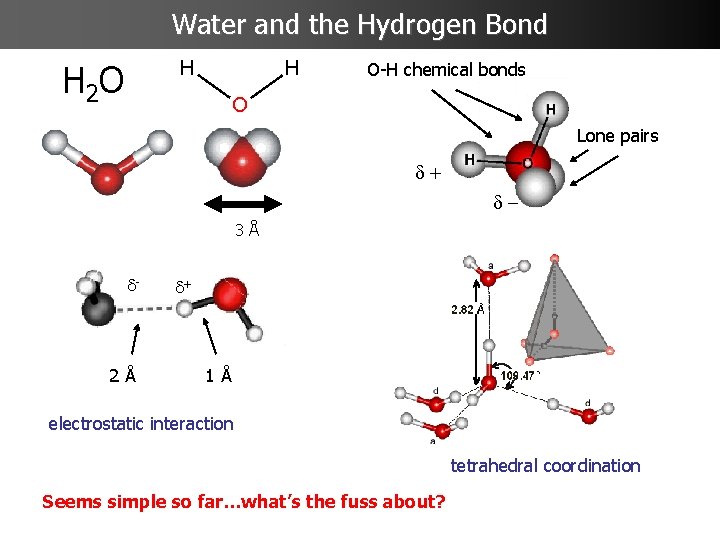
Water and the Hydrogen Bond H 2 O O-H chemical bonds Lone pairs d+ d 3Å d- 2Å d+ 1Å electrostatic interaction tetrahedral coordination Seems simple so far…what’s the fuss about?

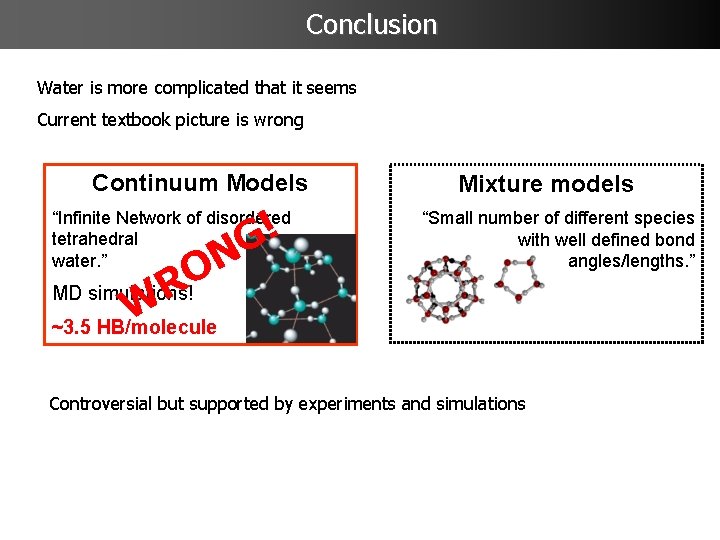
The Controversy: Mixture vs. Continuum Model Ice Tetrahedral structure Old debate prior to 1980 Two extreme models for water Mixture models “Small number of different species with well defined bond angles/lengths. ” Continuum Models “Infinite Network of disordered tetrahedral water. ” MD simulations! ~3. 5 HB/molecule Röntgen 1892 Mostly accepted picture

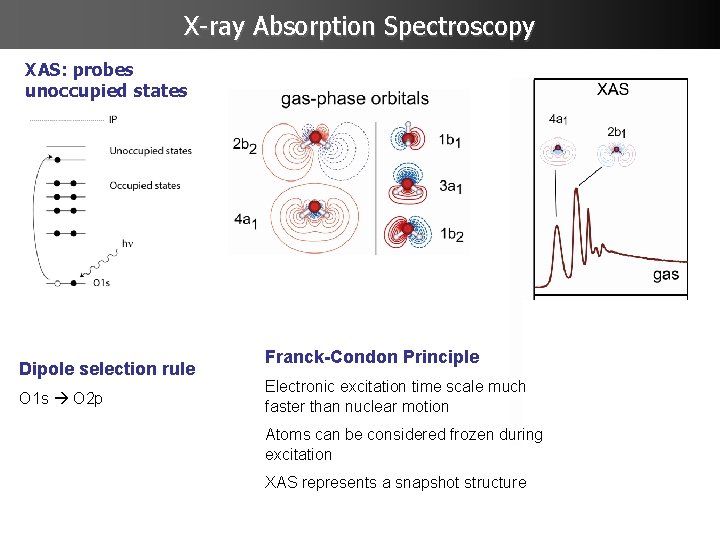
X-ray Absorption Spectroscopy XAS: probes unoccupied states Dipole selection rule O 1 s O 2 p Franck-Condon Principle Electronic excitation time scale much faster than nuclear motion Atoms can be considered frozen during excitation XAS represents a snapshot structure

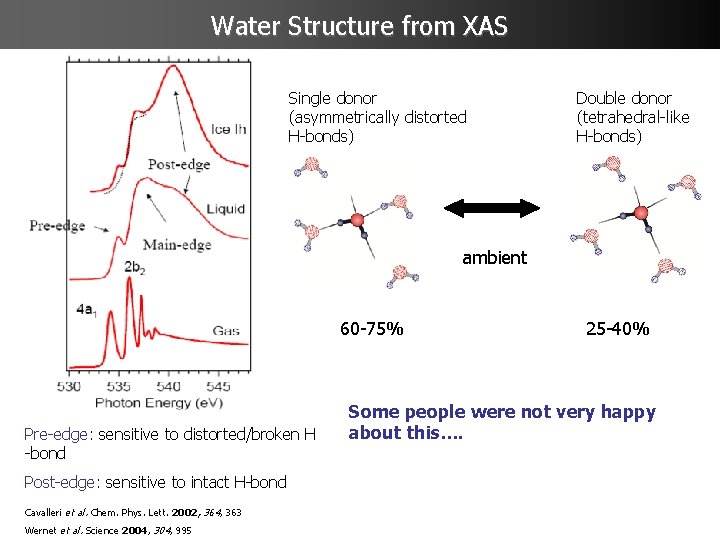
Water Structure from XAS Single donor (asymmetrically distorted H-bonds) Double donor (tetrahedral-like H-bonds) ambient 60 -75% Pre-edge: sensitive to distorted/broken H -bond Post-edge: sensitive to intact H-bond Cavalleri et al. Chem. Phys. Lett. 2002, 364, 363 Wernet et al. Science 2004, 304, 995 25 -40% Some people were not very happy about this….

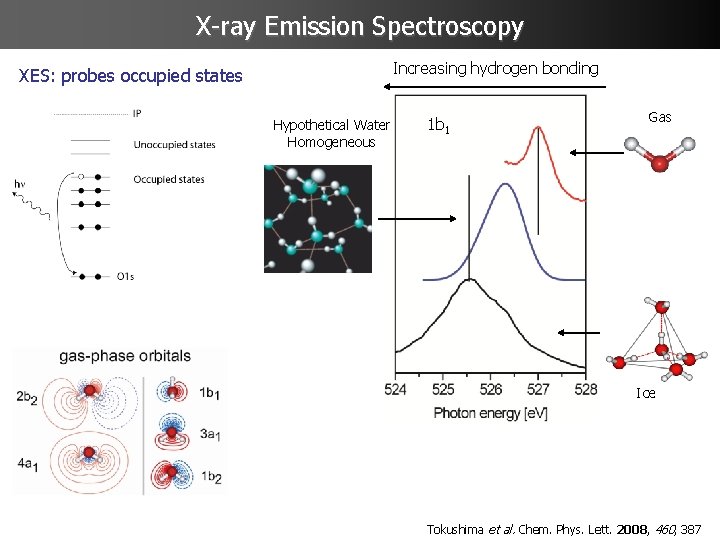
X-ray Emission Spectroscopy Increasing hydrogen bonding XES: probes occupied states Hypothetical Water Homogeneous 1 b 1 Gas Ice Tokushima et al. Chem. Phys. Lett. 2008, 460, 387

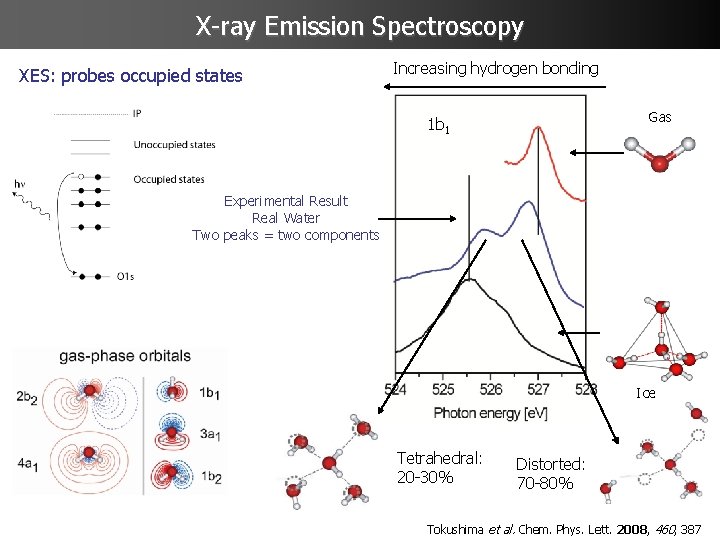
X-ray Emission Spectroscopy XES: probes occupied states Increasing hydrogen bonding Gas 1 b 1 Experimental Result Real Water Two peaks = two components Ice Tetrahedral: 20 -30% Distorted: 70 -80% Tokushima et al. Chem. Phys. Lett. 2008, 460, 387

Small Angle X-ray Scattering Probe for density variations in liquids on the nanometer scale

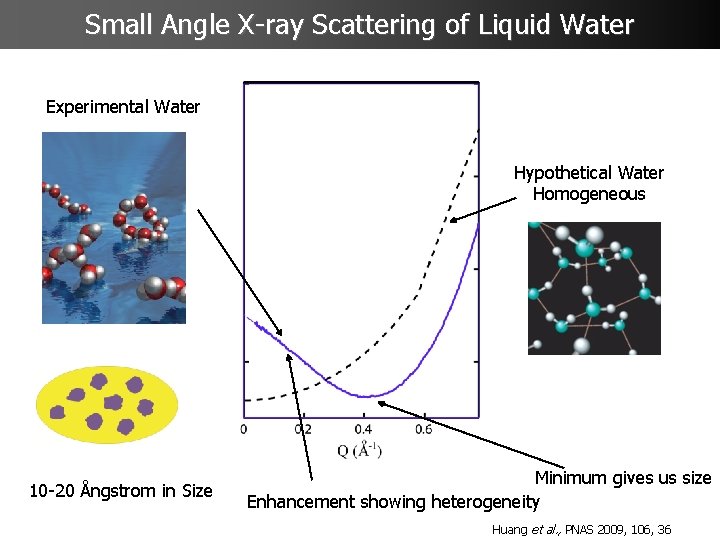
Small Angle X-ray Scattering of Liquid Water Experimental Water Hypothetical Water Homogeneous 10 -20 Ångstrom in Size Minimum gives us size Enhancement showing heterogeneity Huang et al. , PNAS 2009, 106, 36

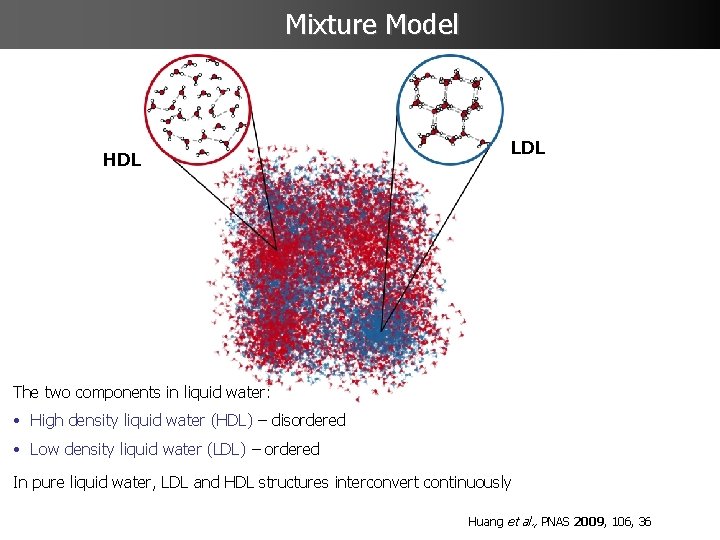
Mixture Model HDL LDL The two components in liquid water: • High density liquid water (HDL) – disordered • Low density liquid water (LDL) – ordered In pure liquid water, LDL and HDL structures interconvert continuously Huang et al. , PNAS 2009, 106, 36

What happens when the H-bond network is disrupted? e. g. temperature increase, addition of salt

Temperature Dependence XAS Increased pre-edge and main edge, decreased post-edge Double donor (LDL) converted to single donor (HDL) H-bond breaking Existing HDL thermally excited (becomes more gas-like) XES Ratio of 1 b 1” to 1 b 1’ peak increase Consistent with XAS (LDL converted to HDL) 1 b 1” peak shifts closer to gas phase Also consistent with XAS (HDL thermally excited, more gas-like) Huang et al. , PNAS 2009, 106, 36

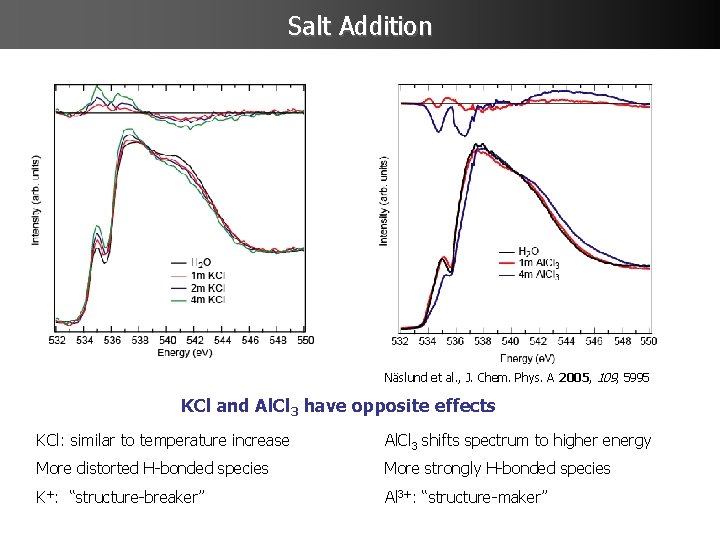
Salt Addition Näslund et al. , J. Chem. Phys. A 2005, 109, 5995 KCl and Al. Cl 3 have opposite effects KCl: similar to temperature increase Al. Cl 3 shifts spectrum to higher energy More distorted H-bonded species More strongly H-bonded species K+: “structure-breaker” Al 3+: “structure-maker”

Conclusion Water is more complicated that it seems Current textbook picture is wrong Continuum Models ! G N “Infinite Network of disordered tetrahedral water. ” O MD simulations! R W ~3. 5 HB/molecule Mixture models “Small number of different species with well defined bond angles/lengths. ” Controversial but supported by experiments and simulations
 Ira nilsson
Ira nilsson Synchrotron radiation
Synchrotron radiation Felexit
Felexit Synchrotron radiation
Synchrotron radiation Polythechnique
Polythechnique Synchrotron
Synchrotron Synchrotron radiation
Synchrotron radiation Synchrotron radiation
Synchrotron radiation Photon density formula
Photon density formula Light: science & applications
Light: science & applications Stanford
Stanford Synchrotron radiation
Synchrotron radiation Synchrotron radiation
Synchrotron radiation Water and water and water water
Water and water and water water Suhanan
Suhanan Mangesti waluyo sedjati
Mangesti waluyo sedjati National synchrotron light source ii
National synchrotron light source ii Psi synchrotron
Psi synchrotron Synchrotron
Synchrotron