Water as a Polar Molecule Essential Question How


![Hydrogen bond [H-bond] Ø l l l A hydrogen bond is a non-covalent bond Hydrogen bond [H-bond] Ø l l l A hydrogen bond is a non-covalent bond](https://slidetodoc.com/presentation_image/2da54b7f69083e8526f95934d58900e4/image-6.jpg)





- Slides: 13

Water as a Polar Molecule Essential Question: How do the unique characteristics of water determine its interactions with chemical and biological systems? TEKS: 8 D

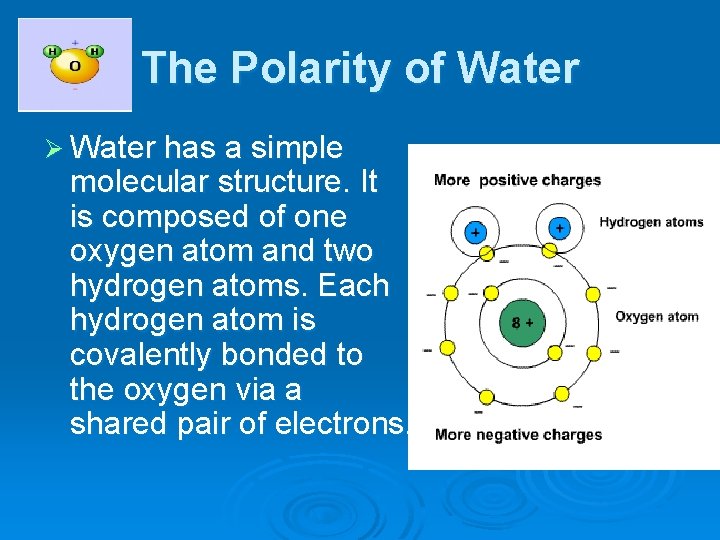
The Polarity of Water Ø Water has a simple molecular structure. It is composed of one oxygen atom and two hydrogen atoms. Each hydrogen atom is covalently bonded to the oxygen via a shared pair of electrons.

Recall Ø What is electronegativity? ? chemical property that describes the ability of an atom (or, more rarely, a functional group) to attract electrons (or electron density) towards itself Ø Looking at water, which element has a higher electronegativity? OXYGEN

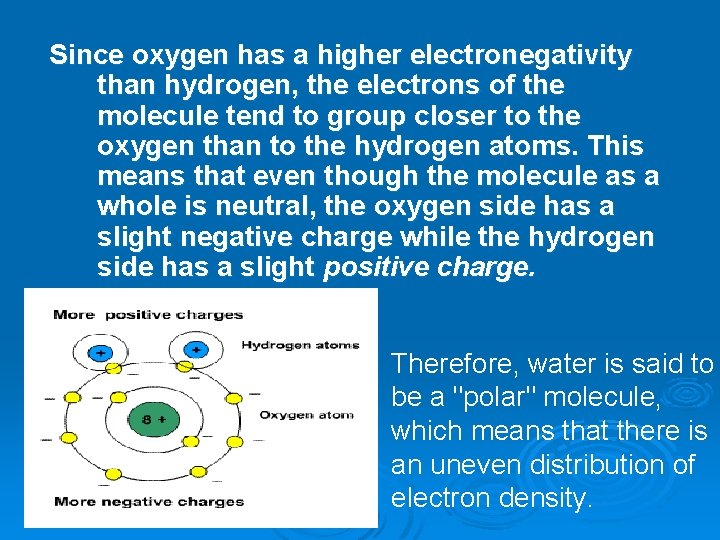
Since oxygen has a higher electronegativity than hydrogen, the electrons of the molecule tend to group closer to the oxygen than to the hydrogen atoms. This means that even though the molecule as a whole is neutral, the oxygen side has a slight negative charge while the hydrogen side has a slight positive charge. Therefore, water is said to be a "polar" molecule, which means that there is an uneven distribution of electron density.

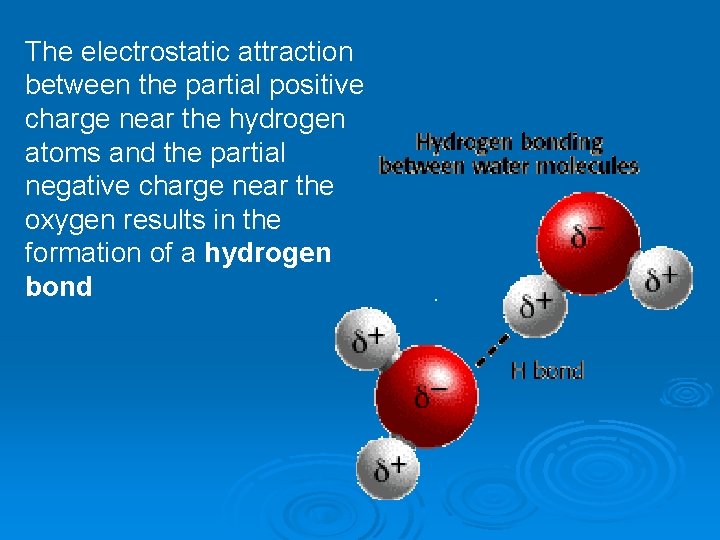
The electrostatic attraction between the partial positive charge near the hydrogen atoms and the partial negative charge near the oxygen results in the formation of a hydrogen bond
![Hydrogen bond Hbond Ø l l l A hydrogen bond is a noncovalent bond Hydrogen bond [H-bond] Ø l l l A hydrogen bond is a non-covalent bond](https://slidetodoc.com/presentation_image/2da54b7f69083e8526f95934d58900e4/image-6.jpg)
Hydrogen bond [H-bond] Ø l l l A hydrogen bond is a non-covalent bond between a partial negative charge and a partial positive charge. Hydrogen bonds tend to be weak. Hydrogen bonds tend to be transient. Hydrogen bonds are very numerous which somewhat offsets their weak and transient nature. On average each water molecule in liquid water is hydrogen bonded to 3. 4 other water molecules.

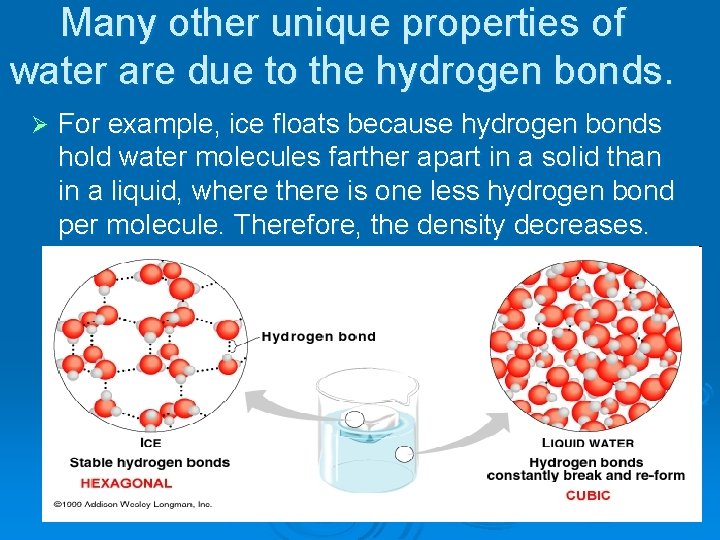
Many other unique properties of water are due to the hydrogen bonds. Ø For example, ice floats because hydrogen bonds hold water molecules farther apart in a solid than in a liquid, where there is one less hydrogen bond per molecule. Therefore, the density decreases.

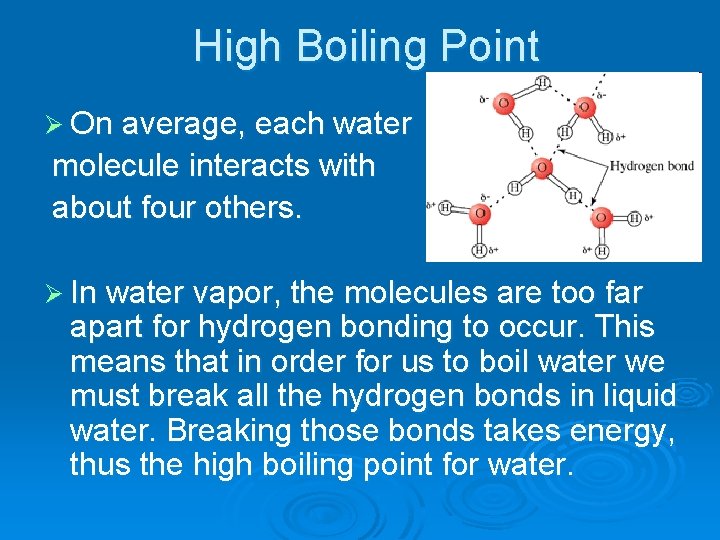
High Boiling Point Ø On average, each water molecule interacts with about four others. Ø In water vapor, the molecules are too far apart for hydrogen bonding to occur. This means that in order for us to boil water we must break all the hydrogen bonds in liquid water. Breaking those bonds takes energy, thus the high boiling point for water.

Cohesion l l The attraction of one water molecule to another resulting from hydrogen bonding. By placing a drop of water on a surface you can directly observe cohesion in the resistance that water droplet shows to wetting, i. e. , water clumps up in a pile despite being a liquid, rather than spreading out over the surface.

Adhesion Ø Similar to cohesion except adhesion involves the attraction of a water molecule to a nonwater molecule. How is adhesion taking place in this image? ?

Surface Tension Ø The water molecules on the surface have partners for hydrogen bonding only within the liquid; above the water surface there are no more molecules available for hydrogen bonding. This means that molecules at the surface experience a net force pulling them inward.


Properties of Water Ø 1. a. Draw the structure of water. Include the partial charges of each atom. Ø b. Why is water considered to be a polar molecule? Ø 2. a. What enables neighboring water molecules to hydrogen-bond to one another? Ø b. How many hydrogen bonds can each water molecule form? Ø 3. Explain the difference between adhesion and cohesion. Give an example of each. Ø 4. What is surface tension? Give an example. Ø 5. Explain why water is considered to have a high boiling point. Ø