Vecchi e nuovi antiangiogenetici Cesare Gridelli Division of






- Slides: 34

Vecchi e nuovi antiangiogenetici Cesare Gridelli Division of Medical Oncology “S. G. Moscati” Hospital – Avellino (Italy) cgridelli@libero. it

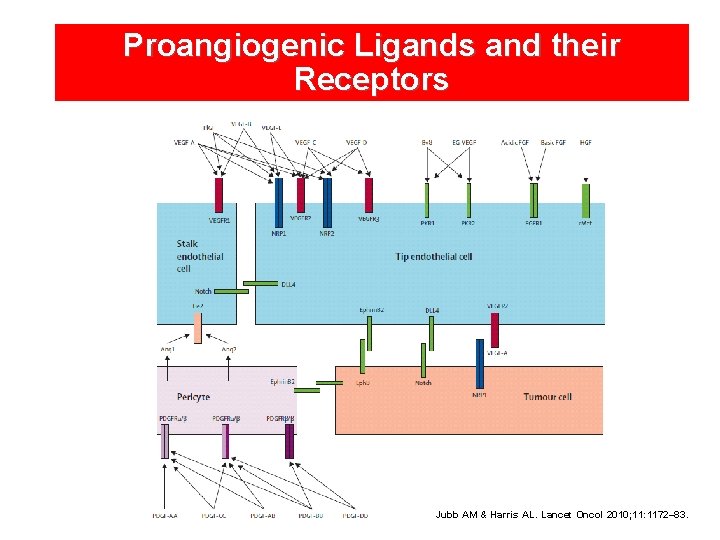
Proangiogenic Ligands and their Receptors Jubb AM & Harris AL. Lancet Oncol 2010; 11: 1172– 83.

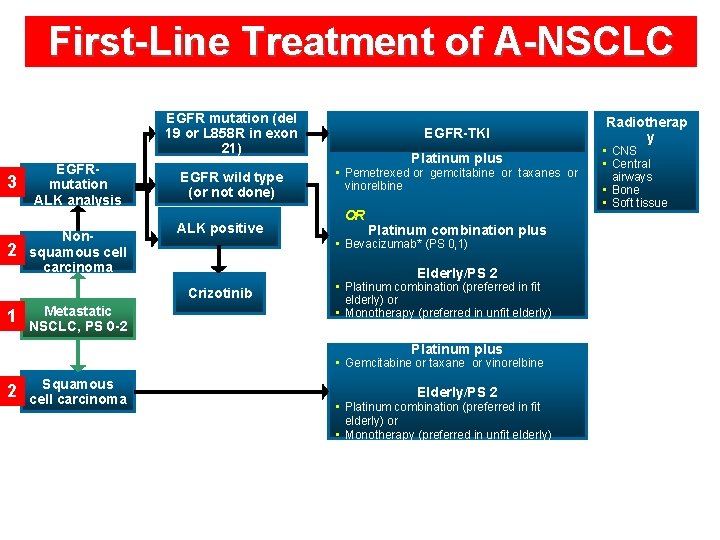
First-Line Treatment of A-NSCLC EGFR mutation (del 19 or L 858 R in exon 21) 3 2 EGFRmutation ALK analysis Nonsquamous cell carcinoma EGFR wild type (or not done) ALK positive Metastatic NSCLC, PS 0 -2 Platinum plus • Pemetrexed or gemcitabine or taxanes or vinorelbine OR Platinum combination plus • Bevacizumab* (PS 0, 1) Elderly/PS 2 Crizotinib 1 EGFR-TKI • Platinum combination (preferred in fit elderly) or • Monotherapy (preferred in unfit elderly) Platinum plus • Gemcitabine or taxane or vinorelbine 2 Squamous cell carcinoma Elderly/PS 2 • Platinum combination (preferred in fit elderly) or • Monotherapy (preferred in unfit elderly) Radiotherap y • CNS • Central airways • Bone • Soft tissue

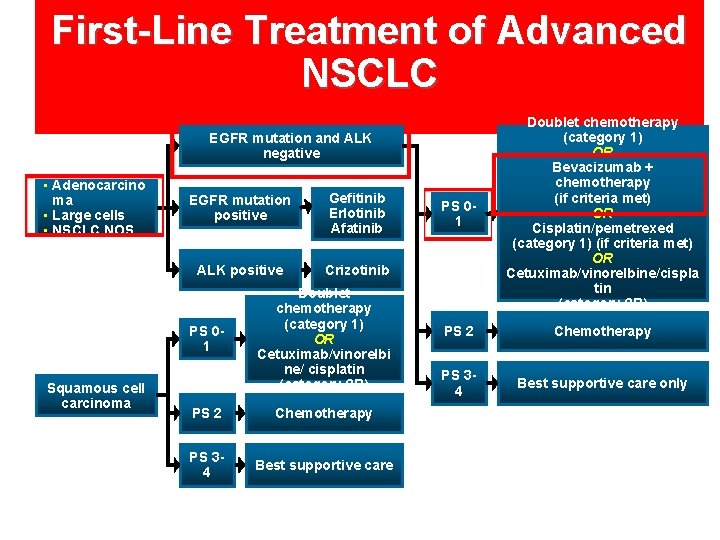
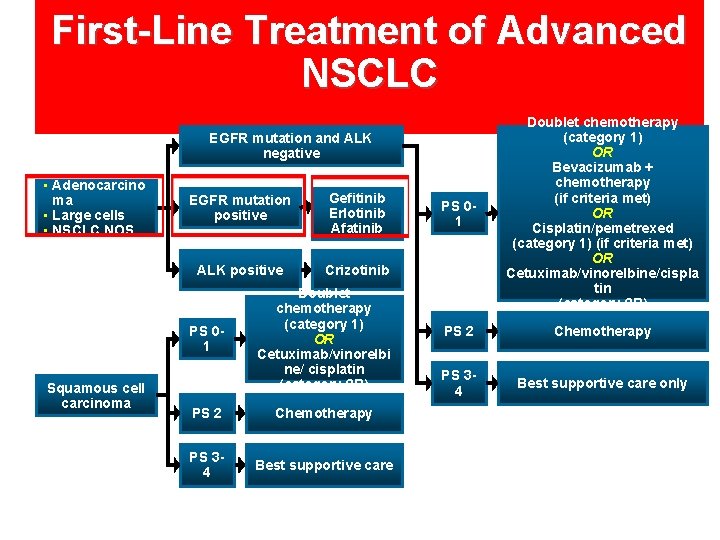
First-Line Treatment of Advanced NSCLC PS 01 Doublet chemotherapy (category 1) OR Bevacizumab + chemotherapy (if criteria met) OR Cisplatin/pemetrexed (category 1) (if criteria met) OR Cetuximab/vinorelbine/cispla tin (category 2 B) PS 2 Chemotherapy PS 34 Best supportive care only EGFR mutation and ALK negative • Adenocarcino ma • Large cells • NSCLC NOS EGFR mutation positive Gefitinib Erlotinib Afatinib ALK positive Crizotinib PS 01 Squamous cell carcinoma Doublet chemotherapy (category 1) OR Cetuximab/vinorelbi ne/ cisplatin (category 2 B) PS 2 Chemotherapy PS 34 Best supportive care

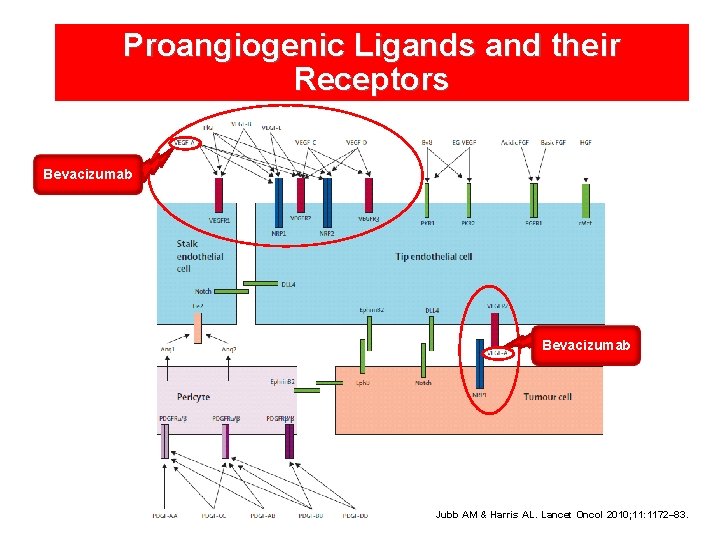
Proangiogenic Ligands and their Receptors Bevacizumab Jubb AM & Harris AL. Lancet Oncol 2010; 11: 1172– 83.

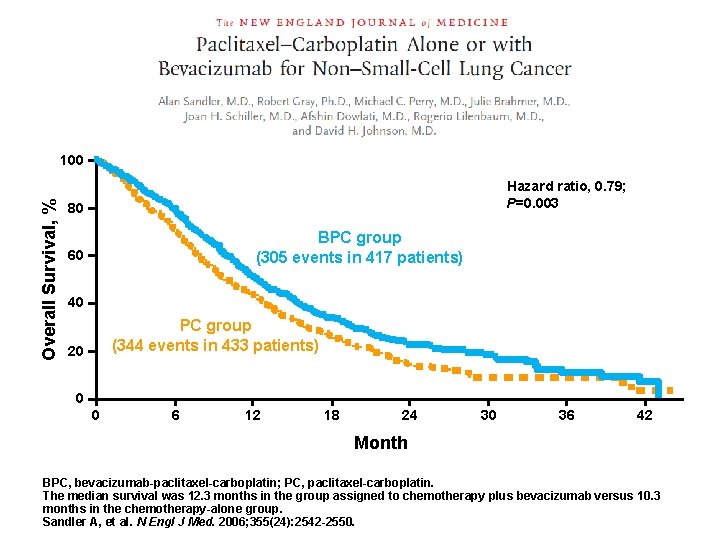
Overall Survival, % 100 Hazard ratio, 0. 79; P=0. 003 80 BPC group (305 events in 417 patients) 60 40 PC group (344 events in 433 patients) 20 0 0 6 12 18 24 30 36 42 Month BPC, bevacizumab-paclitaxel-carboplatin; PC, paclitaxel-carboplatin. The median survival was 12. 3 months in the group assigned to chemotherapy plus bevacizumab versus 10. 3 months in the chemotherapy-alone group. Sandler A, et al. N Engl J Med. 2006; 355(24): 2542 -2550.

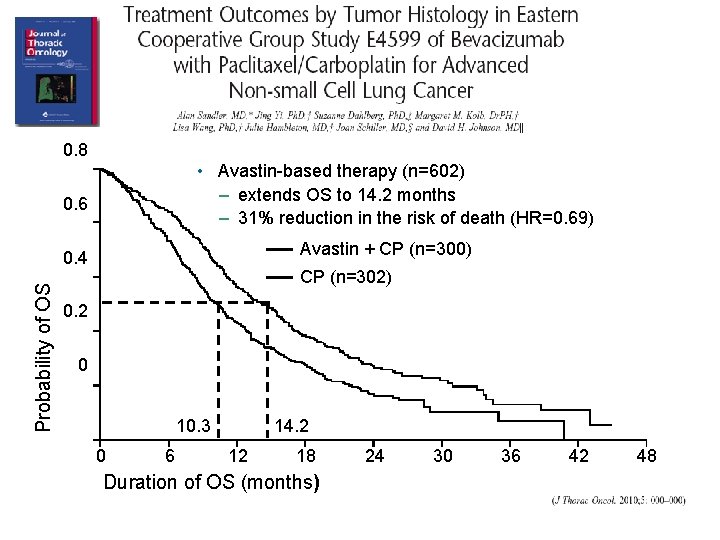
0. 8 • Avastin-based therapy (n=602) – extends OS to 14. 2 months – 31% reduction in the risk of death (HR=0. 69) 0. 6 Avastin + CP (n=300) Probability of OS 0. 4 CP (n=302) 0. 2 0 10. 3 0 6 14. 2 12 18 Duration of OS (months) 24 30 36 42 48

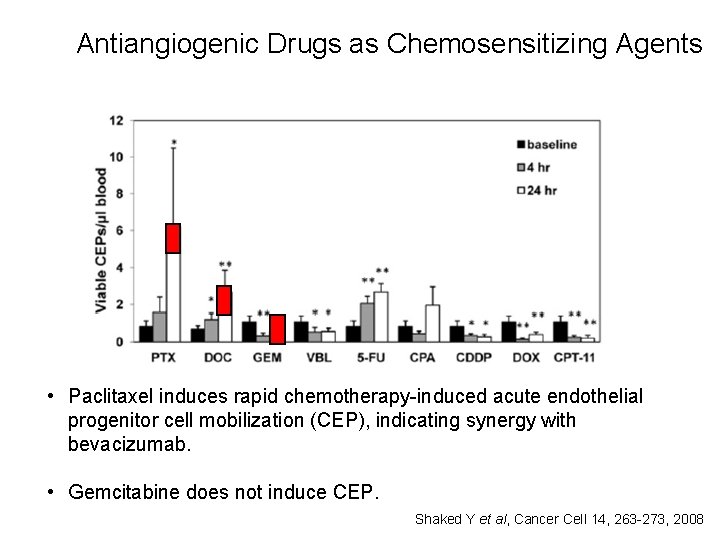
Antiangiogenic Drugs as Chemosensitizing Agents • Paclitaxel induces rapid chemotherapy-induced acute endothelial progenitor cell mobilization (CEP), indicating synergy with bevacizumab. • Gemcitabine does not induce CEP. Shaked Y et al, Cancer Cell 14, 263 -273, 2008

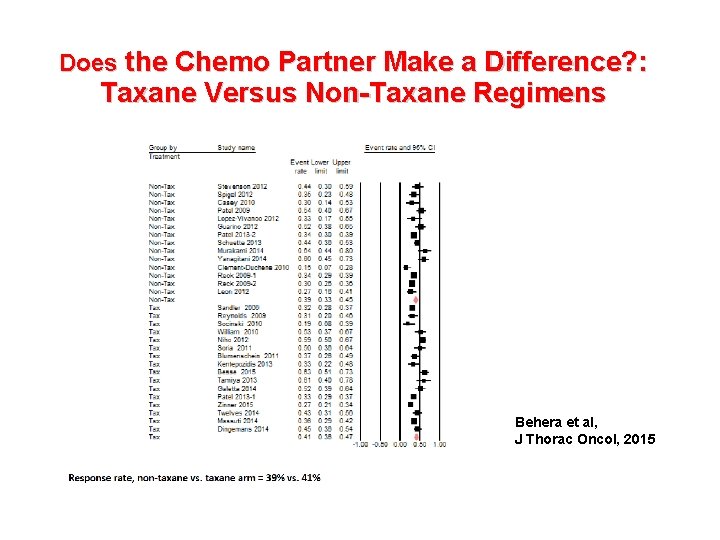
Does the Chemo Partner Make a Difference? : Taxane Versus Non-Taxane Regimens • N=29 studies • N=5890 pts. • Median OS: 14. 4 vs. 13. 7 m, P=0. 5 Behera et al, J Thorac Oncol, 2015

First-Line Treatment of Advanced NSCLC PS 01 Doublet chemotherapy (category 1) OR Bevacizumab + chemotherapy (if criteria met) OR Cisplatin/pemetrexed (category 1) (if criteria met) OR Cetuximab/vinorelbine/cispla tin (category 2 B) PS 2 Chemotherapy PS 34 Best supportive care only EGFR mutation and ALK negative • Adenocarcino ma • Large cells • NSCLC NOS EGFR mutation positive Gefitinib Erlotinib Afatinib ALK positive Crizotinib PS 01 Squamous cell carcinoma Doublet chemotherapy (category 1) OR Cetuximab/vinorelbi ne/ cisplatin (category 2 B) PS 2 Chemotherapy PS 34 Best supportive care

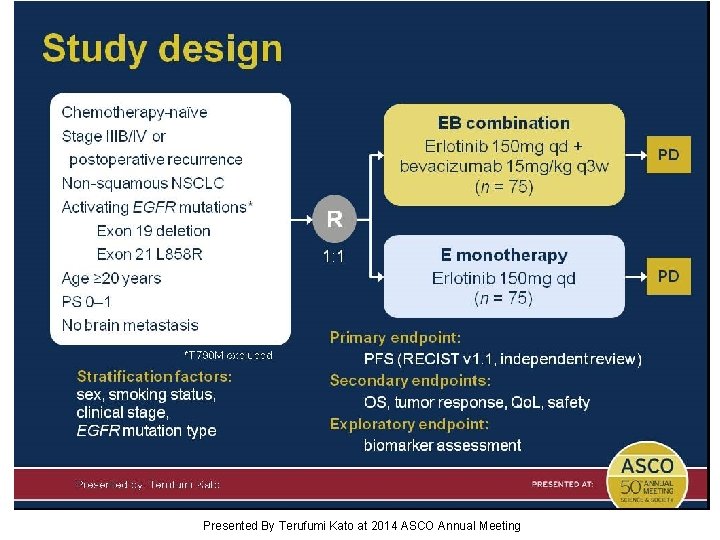
Study design Presented By Terufumi Kato at 2014 ASCO Annual Meeting

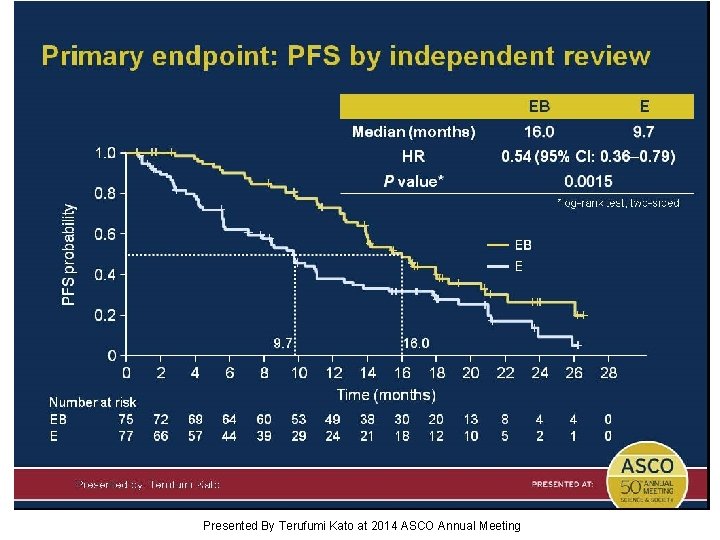
Primary endpoint: PFS by independent review Presented By Terufumi Kato at 2014 ASCO Annual Meeting

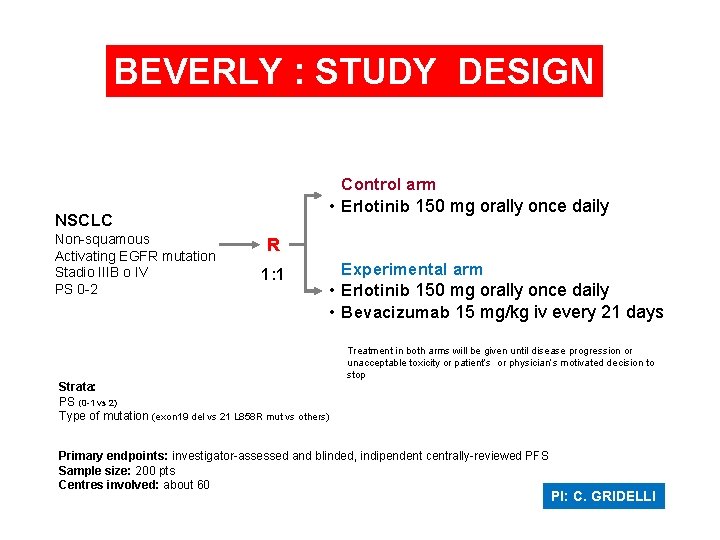
BEVERLY : STUDY DESIGN Control arm • Erlotinib 150 mg orally once daily NSCLC Non-squamous Activating EGFR mutation Stadio IIIB o IV PS 0 -2 R 1: 1 Experimental arm • Erlotinib 150 mg orally once daily • Bevacizumab 15 mg/kg iv every 21 days Treatment in both arms will be given until disease progression or unacceptable toxicity or patient’s or physician’s motivated decision to stop Strata: PS (0 -1 vs 2) Type of mutation (exon 19 del vs 21 L 858 R mut vs others) Primary endpoints: investigator-assessed and blinded, indipendent centrally-reviewed PFS Sample size: 200 pts Centres involved: about 60 PI: C. GRIDELLI

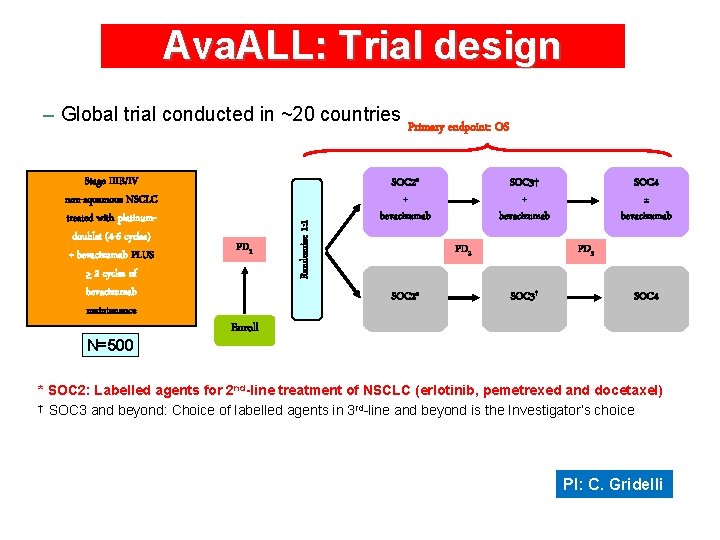
Ava. ALL: Trial design Stage IIIB/IV non-squamous NSCLC treated with platinumdoublet (4 -6 cycles) + bevacizumab PLUS > 2 cycles of bevacizumab maintenance N=500 PD 1 Randomize 1: 1 – Global trial conducted in ~20 countries Primary endpoint: OS SOC 2* + bevacizumab SOC 3† + bevacizumab PD 2 SOC 2* SOC 4 ± bevacizumab PD 3 SOC 3† SOC 4 Enroll * SOC 2: Labelled agents for 2 nd-line treatment of NSCLC (erlotinib, pemetrexed and docetaxel) † SOC 3 and beyond: Choice of labelled agents in 3 rd-line and beyond is the Investigator’s choice PI: C. Gridelli

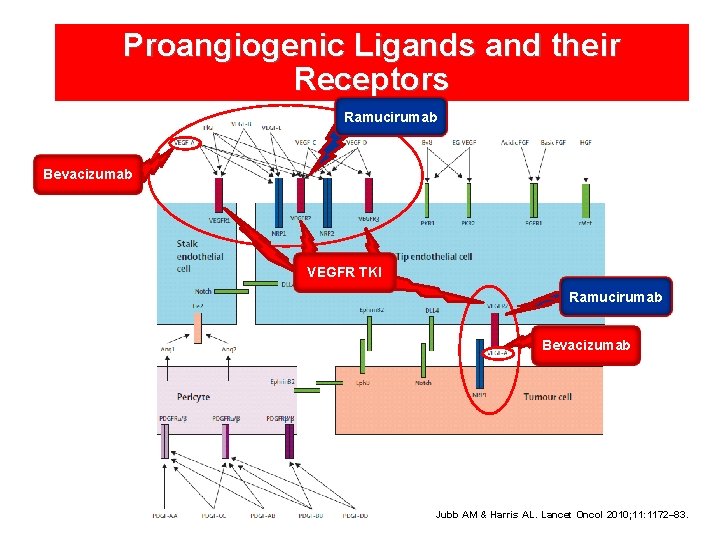
Proangiogenic Ligands and their Receptors Ramucirumab Bevacizumab VEGFR TKI Ramucirumab Bevacizumab Jubb AM & Harris AL. Lancet Oncol 2010; 11: 1172– 83.

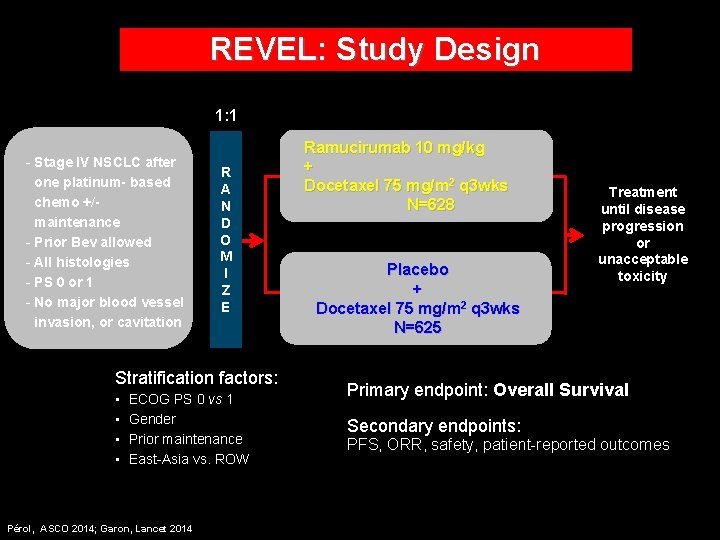
REVEL: Study Design 1: 1 - Stage IV NSCLC after one platinum- based chemo +/maintenance - Prior Bev allowed - All histologies - PS 0 or 1 - No major blood vessel invasion, or cavitation R A N D O M I Z E Stratification factors: • • ECOG PS 0 vs 1 Gender Prior maintenance East-Asia vs. ROW Pérol, ASCO 2014; Garon, Lancet 2014 Ramucirumab 10 mg/kg + Docetaxel 75 mg/m 2 q 3 wks N=628 Placebo + Docetaxel 75 mg/m 2 q 3 wks N=625 Treatment until disease progression or unacceptable toxicity Primary endpoint: Overall Survival Secondary endpoints: PFS, ORR, safety, patient-reported outcomes

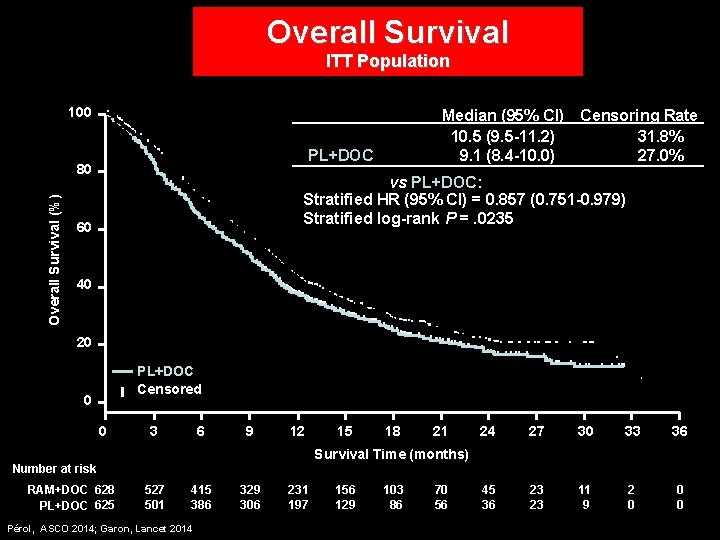
Overall Survival ITT Population 100 RAM+DOC PL+DOC Overall Survival (%) 80 Median (95% CI) Censoring Rate 10. 5 (9. 5 -11. 2) 31. 8% 27. 0% 9. 1 (8. 4 -10. 0) RAM+DOC vs PL+DOC: Stratified HR (95% CI) = 0. 857 (0. 751 -0. 979) Stratified log-rank P =. 0235 60 40 20 RAM+DOC PL+DOC Censored 0 0 3 6 9 12 15 18 21 24 27 30 33 36 45 36 23 23 11 9 2 0 0 0 Survival Time (months) Number at risk RAM+DOC 628 PL+DOC 625 527 501 415 386 Pérol, ASCO 2014; Garon, Lancet 2014 329 306 231 197 156 129 103 86 70 56

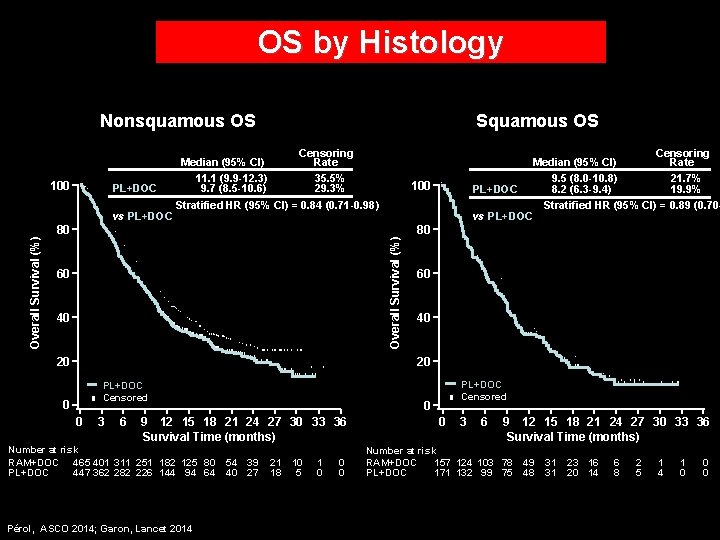
OS by Histology Nonsquamous OS Censoring Median (95% CI) Rate RAM+DOC 11. 1 (9. 9 -12. 3) 35. 5% PL+DOC 9. 7 (8. 5 -10. 6) 29. 3% RAM+DOC Stratified HR (95% CI) = 0. 84 (0. 71 -0. 98) vs PL+DOC 100 80 60 40 20 Censoring Median (95% CI) Rate RAM+DOC 9. 5 (8. 0 -10. 8) 21. 7% PL+DOC 8. 2 (6. 3 -9. 4) 19. 9% RAM+DOC Stratified HR (95% CI) = 0. 89 (0. 70 vs PL+DOC 100 Overall Survival (%) Squamous OS 80 60 40 20 RAM+DOC PL+DOC Censored 0 0 3 6 0 9 12 15 18 21 24 27 30 33 36 Survival Time (months) Number at risk RAM+DOC 465 401 311 251 182 125 80 PL+DOC 447 362 282 226 144 94 64 Pérol, ASCO 2014; Garon, Lancet 2014 RAM+DOC PL+DOC Censored 54 39 40 27 21 18 10 5 1 0 0 3 6 9 12 15 18 21 24 27 30 33 36 Survival Time (months) Number at risk RAM+DOC 157 124 103 78 PL+DOC 171 132 99 75 49 48 31 31 23 16 20 14 6 8 2 5 1 4 1 0 0 0

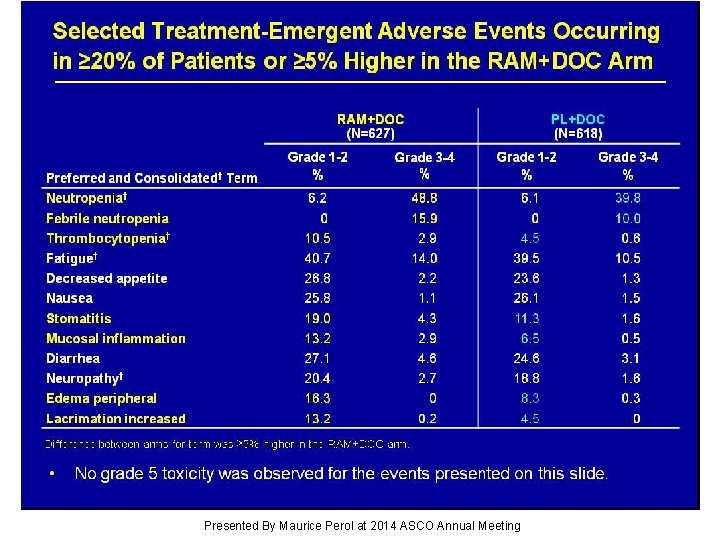
Selected Treatment-Emergent Adverse Events Occurring in ≥ 20% of Patients or ≥ 5% Higher in the RAM+DOC Arm Presented By Maurice Perol at 2014 ASCO Annual Meeting


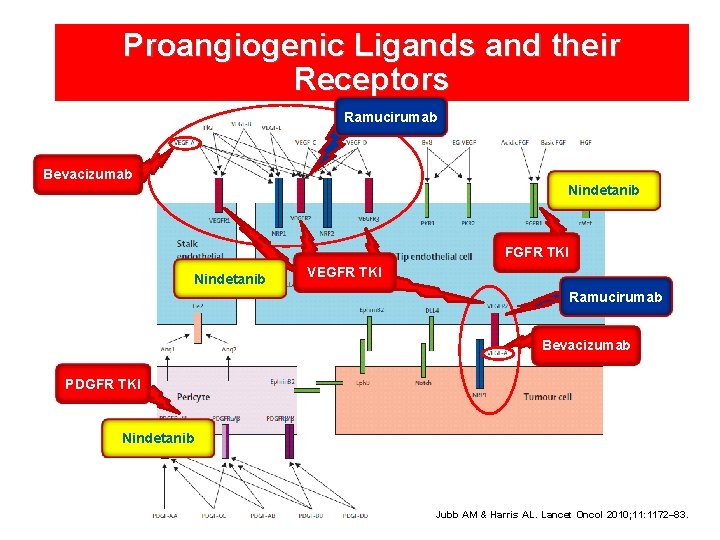
Proangiogenic Ligands and their Receptors Ramucirumab Bevacizumab Nindetanib FGFR TKI Nindetanib VEGFR TKI Ramucirumab Bevacizumab PDGFR TKI Nindetanib Jubb AM & Harris AL. Lancet Oncol 2010; 11: 1172– 83.

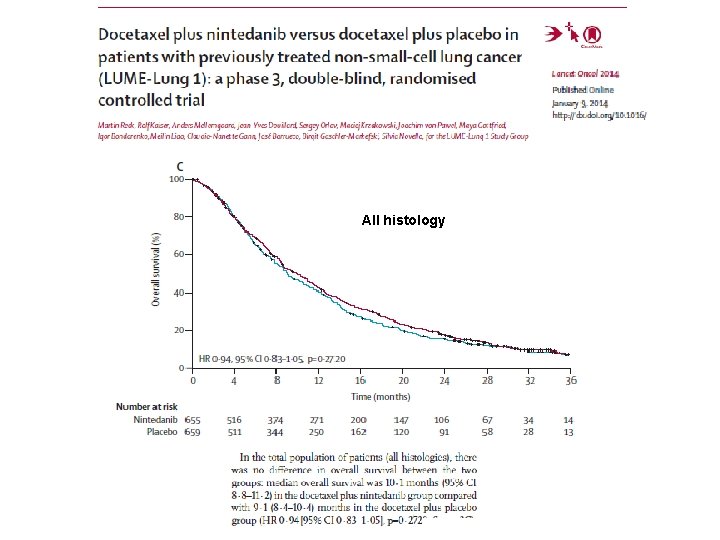
LUME-Lung 1: Randomised Phase III Study Stage IIIB/IV* or recurrent NSCLC patients after first-line chemotherapy (all histologies) R A N D O M I S E PD Placebo BID po, Days 2– 21, + docetaxel 75 mg/m 2 IV, Day 1, 21 -day cycles (n=659) PD 1: 1 N=1314 Primary Endpoint: Nintedanib 200 mg BID po, Days 2– 21 + docetaxel 75 mg/m 2 IV, Day 1, 21 -day cycles (n=655) Number of docetaxel cycles not restricted Monotherapy allowed after ≥ 4 cycles of combination therapy PFS by independent central review Key Secondary Endpoint: OS, prespecified hierarchical analyses of patients with adenocarcinoma who progressed in <9 months after start of first-line therapy, all patients with adenocarcinoma, and ITT population Stratification: ECOG PS (0 vs. 1) Prior bevacizumab (yes vs. no) Histology (squamous vs. non-squamous) Brain metastases (yes vs. no) • Reck M, et al. Lancet Oncol. 2014; 15: 143 -55. Regions: Accrual: Europe/Asia/South Africa 23 Dec 2008 to 09 Feb 2011 22

All histology


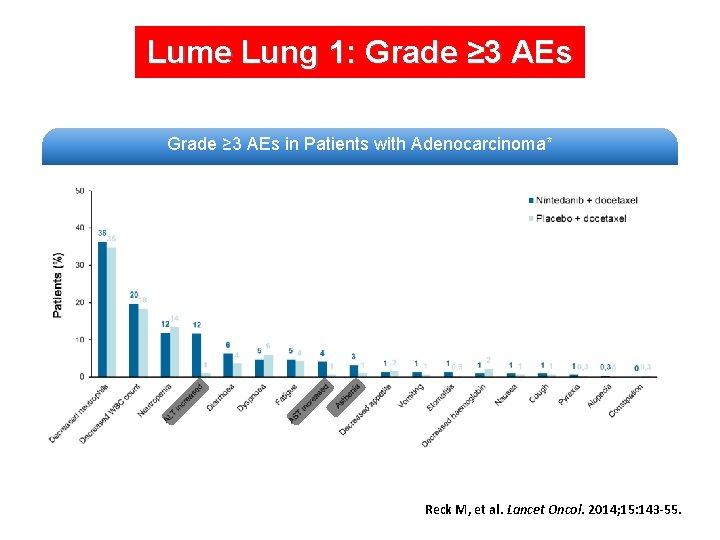
Lume Lung 1: Grade ≥ 3 AEs in Patients with Adenocarcinoma* Reck M, et al. Lancet Oncol. 2014; 15: 143 -55.

Approved by EMA on November 21, 2014 VARGATEF® (nintedanib) is indicated in combination with docetaxel for the treatment of adult patients with locally advanced, metastatic or locally recurrent non small cell lung cancer (NSCLC) of adenocarcinoma tumour histology after first-line chemotherapy

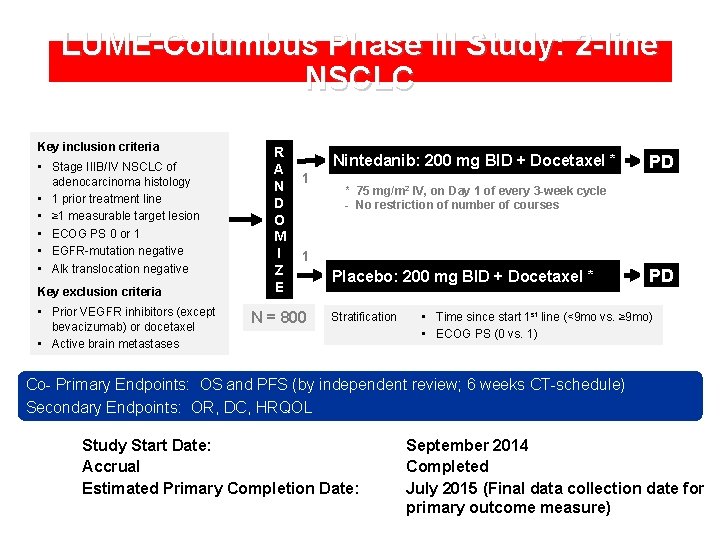
LUME-Columbus Phase III Study: 2 -line NSCLC Key inclusion criteria • Stage IIIB/IV NSCLC of adenocarcinoma histology • 1 prior treatment line • ≥ 1 measurable target lesion • ECOG PS 0 or 1 • EGFR-mutation negative • Alk translocation negative Key exclusion criteria • Prior VEGFR inhibitors (except bevacizumab) or docetaxel • Active brain metastases R A N D O M I Z E Nintedanib: 200 mg BID + Docetaxel * 1 PD * 75 mg/m 2 IV, on Day 1 of every 3 -week cycle - No restriction of number of courses 1 N = 800 Placebo: 200 mg BID + Docetaxel * Stratification PD • Time since start 1 st line (<9 mo vs. ≥ 9 mo) • ECOG PS (0 vs. 1) Co- Primary Endpoints: OS and PFS (by independent review; 6 weeks CT-schedule) Secondary Endpoints: OR, DC, HRQOL Study Start Date: Accrual Estimated Primary Completion Date: September 2014 Completed July 2015 (Final data collection date for primary outcome measure)

Chairmen F. de Marinis C. Gridelli Panelists F. Ciardiello L. Crinò F. de Marinis J. Y. Douillard C. Gridelli F. Griesinger D. Lambrechts M. Perol S. Ramalingam E. Smit

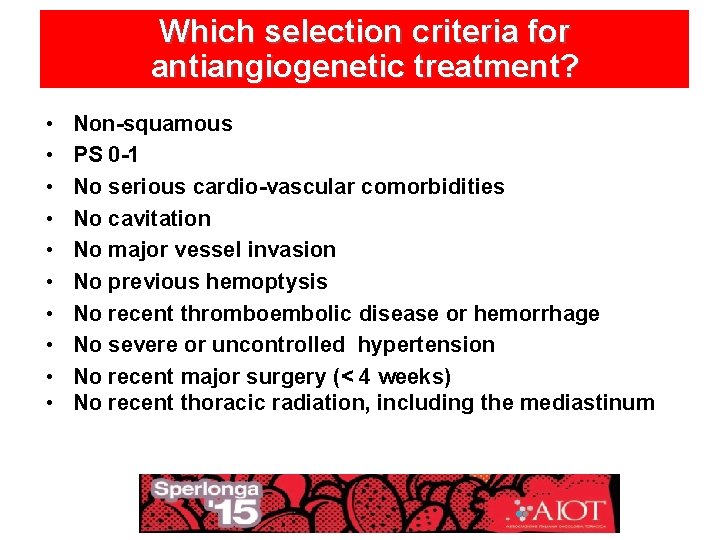
Which selection criteria for antiangiogenetic treatment? • • • Non-squamous PS 0 -1 No serious cardio-vascular comorbidities No cavitation No major vessel invasion No previous hemoptysis No recent thromboembolic disease or hemorrhage No severe or uncontrolled hypertension No recent major surgery (< 4 weeks) No recent thoracic radiation, including the mediastinum

Any predictive biomarker for antiangiogenetic treatment ? • So far, no biomarker for NSCLC patient selection (as well as other cancers) has been validated for clinical practice.

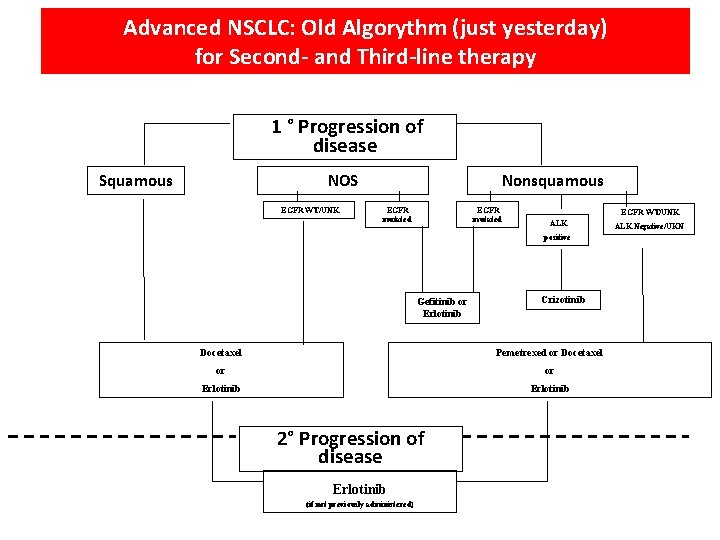
Advanced NSCLC: Old Algorythm (just yesterday) for Second- and Third-line therapy 1 ° Progression of disease Squamous NOS EGFR WT/UNK Nonsquamous EGFR mutated EGFR WT/UNK ALK positive Gefitinib or Erlotinib Crizotinib Docetaxel Pemetrexed or Docetaxel or or Erlotinib 2° Progression of disease Erlotinib (if not previously administered) ALK Negative/UKN

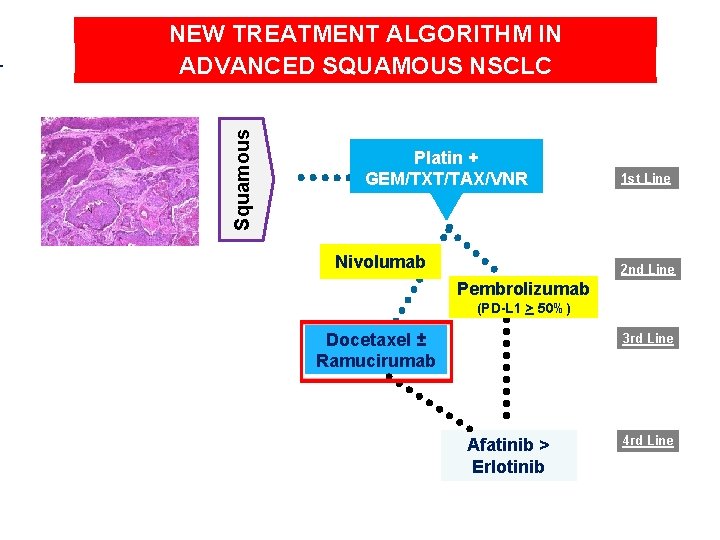
Squamous NEW TREATMENT ALGORITHM IN ADVANCED SQUAMOUS NSCLC Platin + GEM/TXT/TAX/VNR Nivolumab 1 st Line 2 nd Line Pembrolizumab (PD-L 1 > 50%) Docetaxel ± Ramucirumab 3 rd Line Afatinib > Erlotinib 4 rd Line

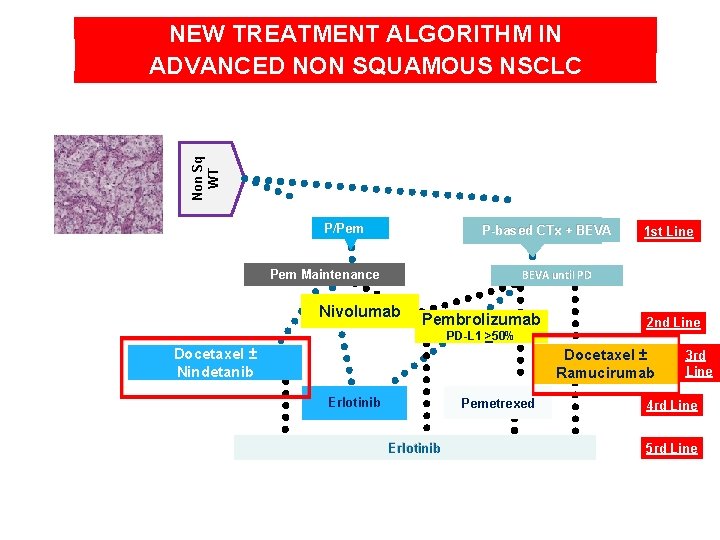
Non Sq WT NEW TREATMENT ALGORITHM IN ADVANCED NON SQUAMOUS NSCLC P/Pem P-based CTx + BEVA 1 st Line BEVA until PD Pem Maintenance Nivolumab Pembrolizumab PD-L 1 >50% Docetaxel ± Nindetanib 2 nd Line Docetaxel ± Ramucirumab Erlotinib Pemetrexed Erlotinib 3 rd Line 4 rd Line 5 rd Line

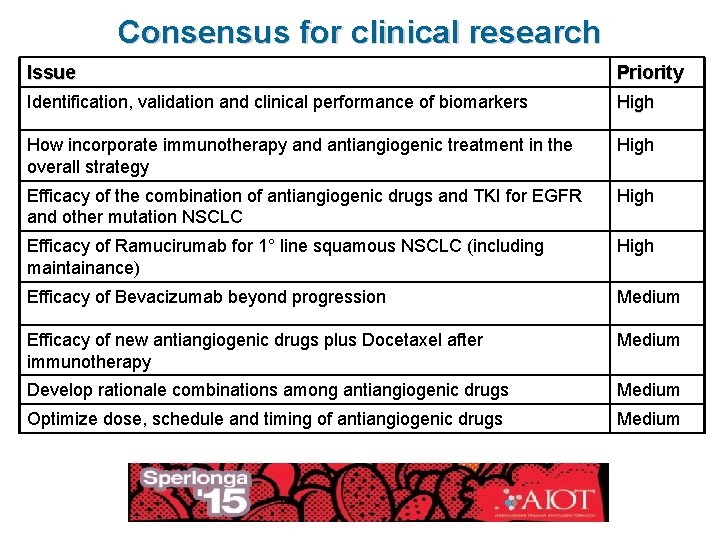
Consensus for clinical research Issue Priority Identification, validation and clinical performance of biomarkers High How incorporate immunotherapy and antiangiogenic treatment in the overall strategy High Efficacy of the combination of antiangiogenic drugs and TKI for EGFR and other mutation NSCLC High Efficacy of Ramucirumab for 1° line squamous NSCLC (including maintainance) High Efficacy of Bevacizumab beyond progression Medium Efficacy of new antiangiogenic drugs plus Docetaxel after immunotherapy Medium Develop rationale combinations among antiangiogenic drugs Medium Optimize dose, schedule and timing of antiangiogenic drugs Medium
 Amilcare angelo vecchi
Amilcare angelo vecchi Metonimia
Metonimia Manuela vecchi
Manuela vecchi Giancarlo vecchi polimi
Giancarlo vecchi polimi Nuovi antidiabetici orali
Nuovi antidiabetici orali Neoclassicismo slide
Neoclassicismo slide Nuovi prodotti alimentari
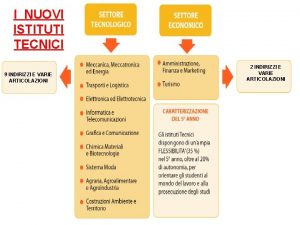
Nuovi prodotti alimentari Nuovi istituti tecnici
Nuovi istituti tecnici Nuovi prodotti alimentari
Nuovi prodotti alimentari Nuovi percorsi professionali
Nuovi percorsi professionali Vino nuovo in otri nuovi
Vino nuovo in otri nuovi Nuovi antidiabetici orali
Nuovi antidiabetici orali Indirizzi istituti tecnici
Indirizzi istituti tecnici Billy cesare
Billy cesare Silvio cesare
Silvio cesare Disco cifrante da stampare
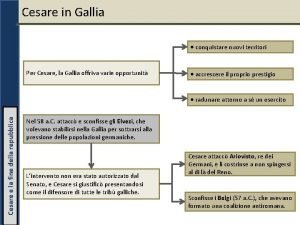
Disco cifrante da stampare Gallia ai tempi di cesare
Gallia ai tempi di cesare Scuola media giulio cesare savignano
Scuola media giulio cesare savignano Cesare fieschi
Cesare fieschi Cesare22
Cesare22 Ipia cesare pesenti
Ipia cesare pesenti Nick iyer
Nick iyer Dott cesare burti
Dott cesare burti Ipia cesare pesenti
Ipia cesare pesenti Centro studi cesare ferrari
Centro studi cesare ferrari Caio giulio cesare osimo
Caio giulio cesare osimo Scuola di scienze politiche cesare alfieri
Scuola di scienze politiche cesare alfieri Microlearning rivoltella
Microlearning rivoltella Chapter 6 section 2 the enlightenment in europe
Chapter 6 section 2 the enlightenment in europe Cesare sconfigge e insegue gli elvezi
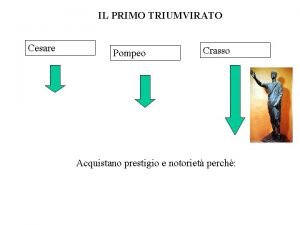
Cesare sconfigge e insegue gli elvezi Triumvirato cesare pompeo crasso
Triumvirato cesare pompeo crasso Istituto comprensivo caio giulio cesare osimo
Istituto comprensivo caio giulio cesare osimo Cesare grazioli
Cesare grazioli Pavese mappa concettuale
Pavese mappa concettuale Preolium
Preolium