Variola and Monkeypox Viruses Utilize Conserved Mechanisms of







- Slides: 26

Variola and Monkeypox Viruses Utilize Conserved Mechanisms of Virion Motility and Release That Depend on Abl and Src Family Tyrosine Kinases Patrick M. Reeves, Scott K. Smith, Victoria A. Olson, Steve H. Thorne, William Bornmann, Inger K. Damon, and Daniel Kalman Tiffany Ha Idris Hooper Fei Yi

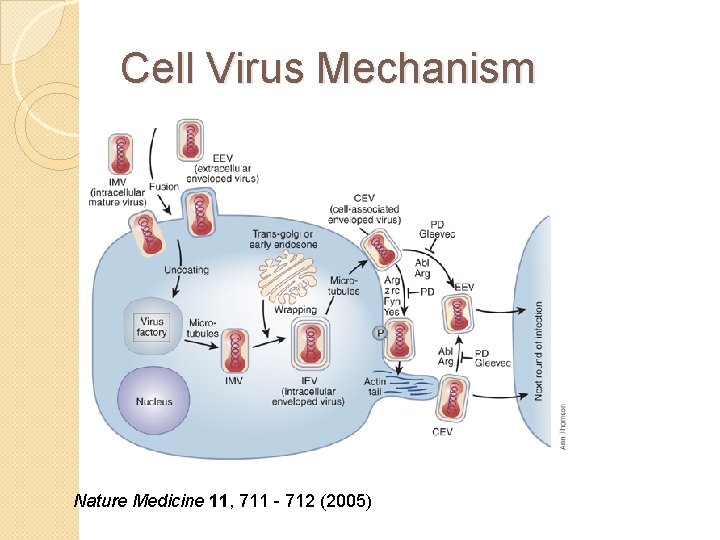
Cell Virus Mechanism Nature Medicine 11, 711 - 712 (2005)

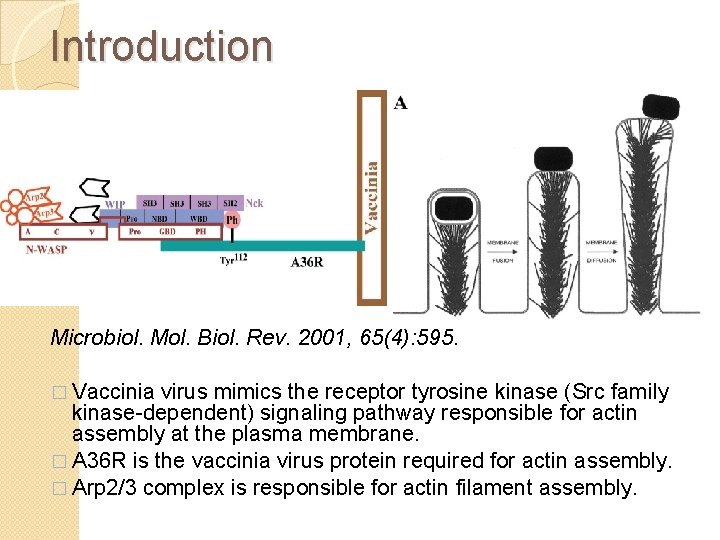
Introduction Microbiol. Mol. Biol. Rev. 2001, 65(4): 595. � Vaccinia virus mimics the receptor tyrosine kinase (Src family kinase-dependent) signaling pathway responsible for actin assembly at the plasma membrane. � A 36 R is the vaccinia virus protein required for actin assembly. � Arp 2/3 complex is responsible for actin filament assembly.

Mobility of cells induced by Vaccinia Virus 2007 Cell Motility Lab Dr. Michael Way, Cell Motility Laboratory. Cancer Research U London Research Institute

Purpose �Determine similarities between Vac. V, Var. V, and MPX actin tail formation �Determine the tyrosine kinase inhibitors and its effect of the actin tail formation and polymerization �Effects of the tyrosine kinase inhibitors on the infectivity of the virus

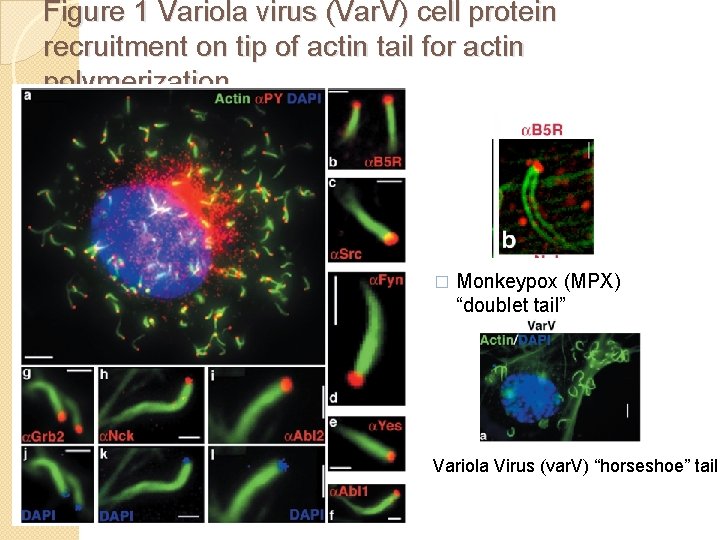
Figure 1 Variola virus (Var. V) cell protein recruitment on tip of actin tail for actin polymerization � Monkeypox (MPX) “doublet tail” Variola Virus (var. V) “horseshoe” tail

Var. V and MPX analogous to Vaccinia virus localizing the cellular proteins to actin tail 2007 Cell Motility Lab Dr. Michael Way, Cell Motility Laboratory. Cancer Research UK London Research Institute

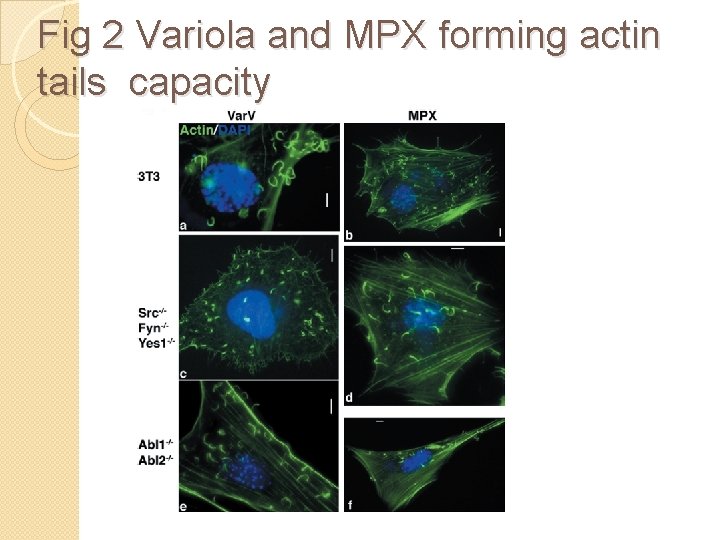
Fig 2 Variola and MPX forming actin tails capacity

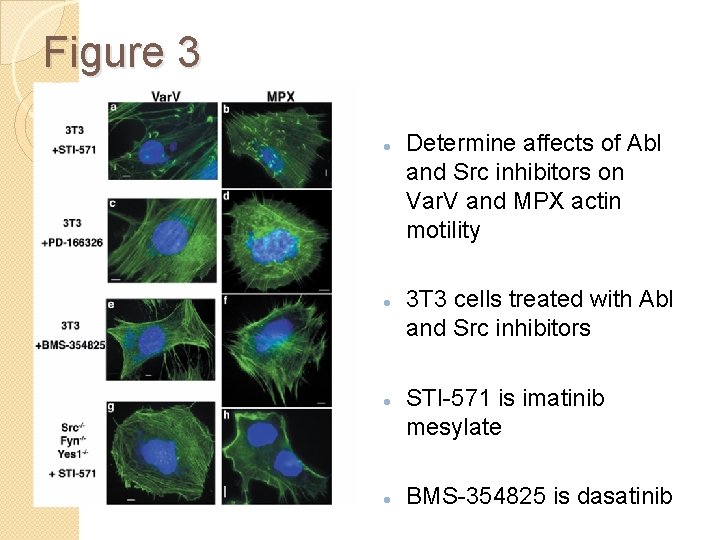
Figure 3 Determine affects of Abl and Src inhibitors on Var. V and MPX actin motility 3 T 3 cells treated with Abl and Src inhibitors STI-571 is imatinib mesylate BMS-354825 is dasatinib

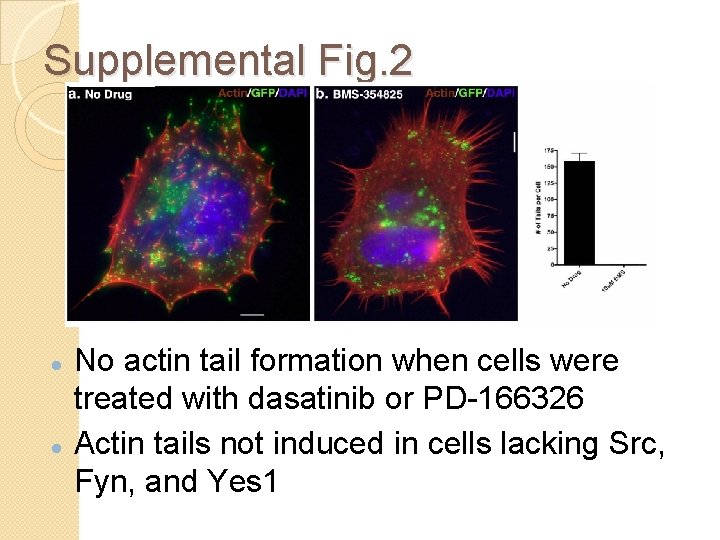
Supplemental Fig. 2 No actin tail formation when cells were treated with dasatinib or PD-166326 Actin tails not induced in cells lacking Src, Fyn, and Yes 1

Fig. 3 and S 2 Conclusion Varv and MPX utilize Abl or Src family tyrosine kinases to form actin tails Functionally redundant usage of kinases by Varv and MXP

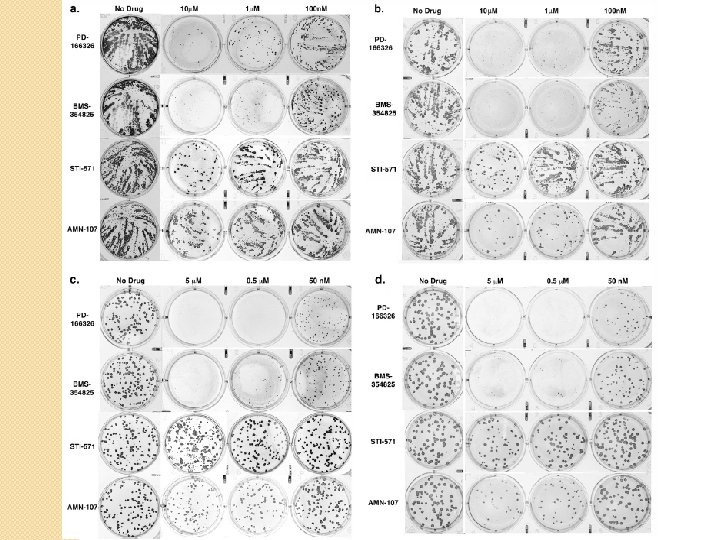
Figure 4 Tested the effects of tyrosine kinase inhibitors on plaque and “comet” formation “Comets” are indicators of released EEV Comet and CMC assays were performed BSC-40 cells treated with various concentrations of inhibitors; stained with poxvirus Pab


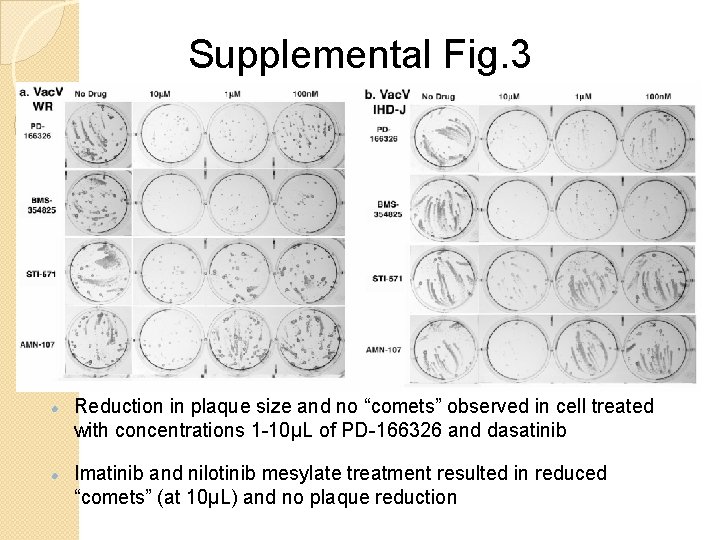
Supplemental Fig. 3 Reduction in plaque size and no “comets” observed in cell treated with concentrations 1 -10μL of PD-166326 and dasatinib Imatinib and nilotinib mesylate treatment resulted in reduced “comets” (at 10μL) and no plaque reduction

Figure 4 and S 3 Conclusion Inhibiting Abl family kinases reduced EEV, but not CEV, produced by Var. V, MPX, and Vac. V Inhibiting Src family kinases eliminated or reduced Var. V. MPX, and Vac. V replication

Figure 5 a � BSH and SLN are 2 strains of Var. V � BSC-40 cells were infected with virus at MOI 0. 1 � Drugs were added post infection � Viral supernatant was harvested (EEV) � # of EEV was determined by plaque enumeration

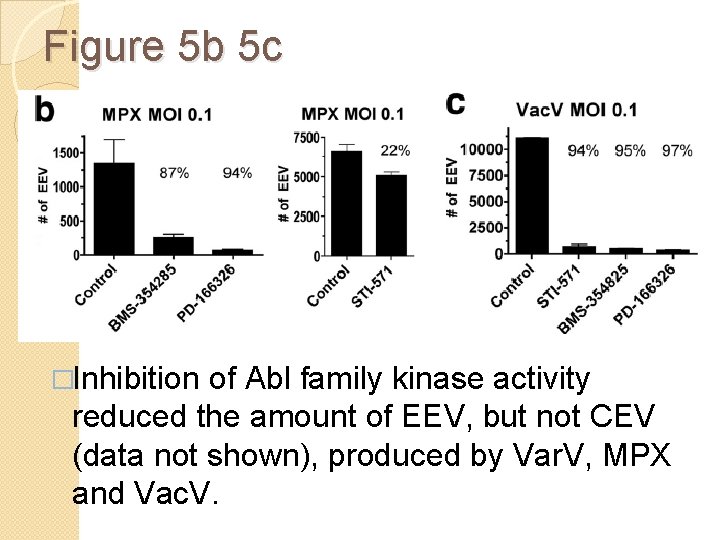
Figure 5 b 5 c �Inhibition of Abl family kinase activity reduced the amount of EEV, but not CEV (data not shown), produced by Var. V, MPX and Vac. V.

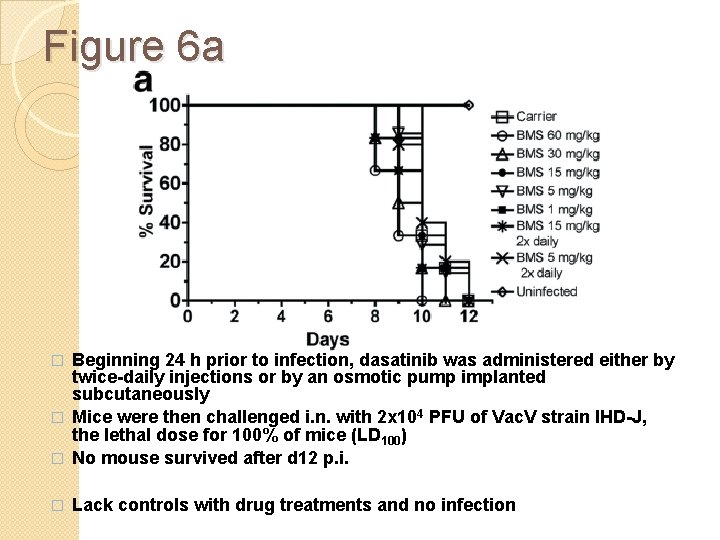
Figure 6 a Beginning 24 h prior to infection, dasatinib was administered either by twice-daily injections or by an osmotic pump implanted subcutaneously � Mice were then challenged i. n. with 2 x 104 PFU of Vac. V strain IHD-J, the lethal dose for 100% of mice (LD 100) � No mouse survived after d 12 p. i. � � Lack controls with drug treatments and no infection

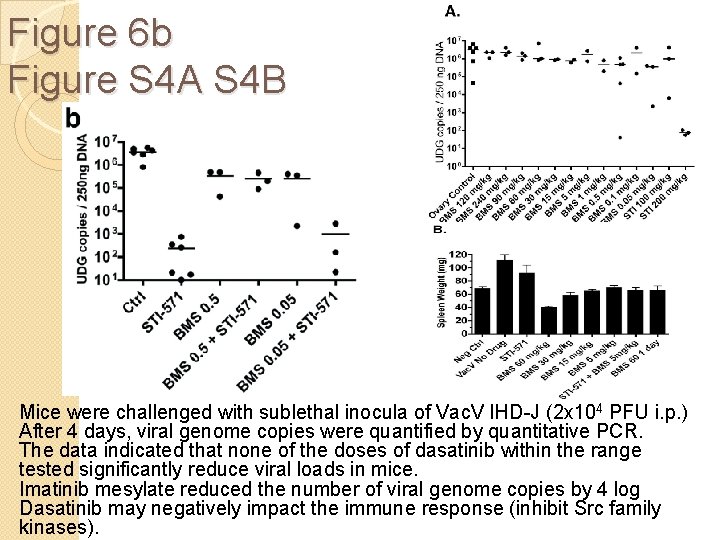
Figure 6 b Figure S 4 A S 4 B Mice were challenged with sublethal inocula of Vac. V IHD-J (2 x 104 PFU i. p. ) After 4 days, viral genome copies were quantified by quantitative PCR. The data indicated that none of the doses of dasatinib within the range tested significantly reduce viral loads in mice. Imatinib mesylate reduced the number of viral genome copies by 4 log Dasatinib may negatively impact the immune response (inhibit Src family kinases).

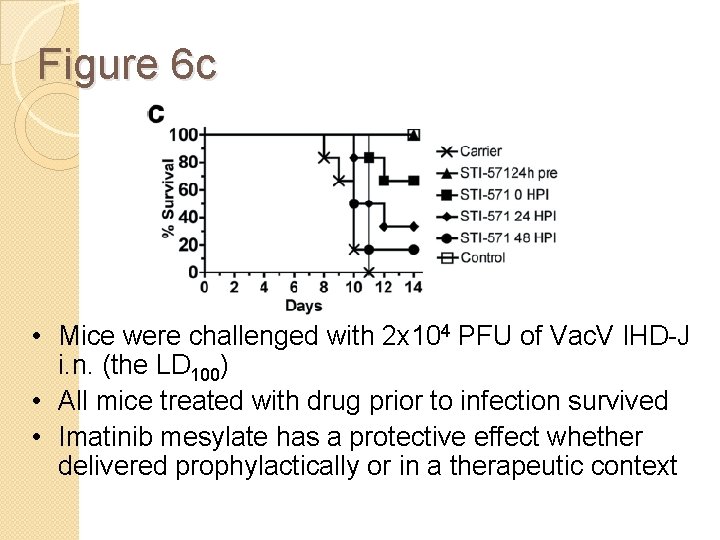
Figure 6 c • Mice were challenged with 2 x 104 PFU of Vac. V IHD-J i. n. (the LD 100) • All mice treated with drug prior to infection survived • Imatinib mesylate has a protective effect whether delivered prophylactically or in a therapeutic context

Figure 6 d Whether imatinib mesylate interfered with the acquisition of protective immune memory? Mice previously challenged with the LD 100 and treated with imatinib mesylate were allowed to rest for 10 to 12 weeks Naïve mice all succumbed within 4 to 9 days, whereas all imatinib mesylate survivors and immunized mice remained viable. Together, these data indicate that administration of imatinib mesylate does not interfere with the acquisition of protective immune memory.

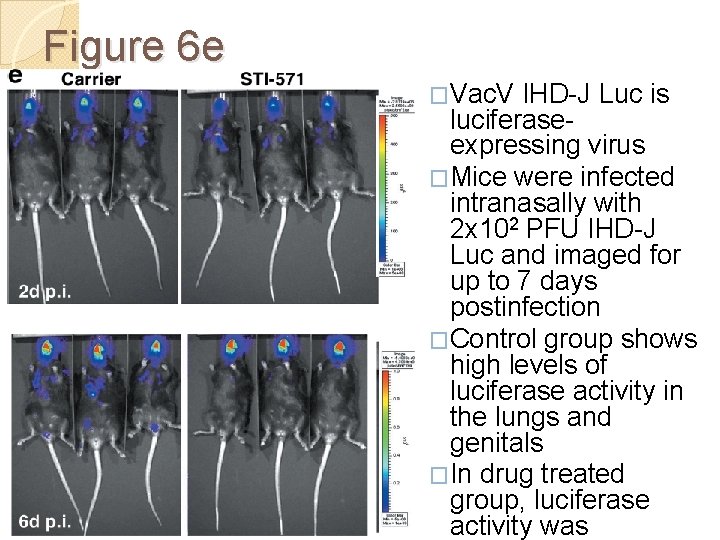
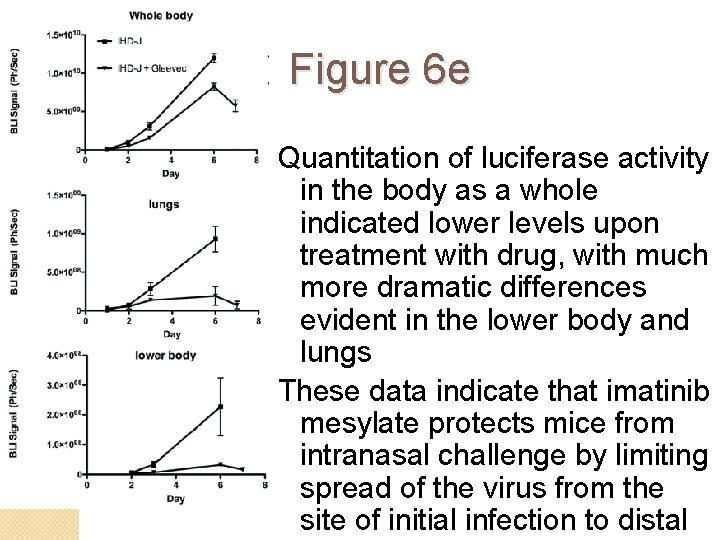
Figure 6 e �Vac. V IHD-J Luc is luciferaseexpressing virus �Mice were infected intranasally with 2 x 102 PFU IHD-J Luc and imaged for up to 7 days postinfection �Control group shows high levels of luciferase activity in the lungs and genitals �In drug treated group, luciferase activity was

Figure 6 e Quantitation of luciferase activity in the body as a whole indicated lower levels upon treatment with drug, with much more dramatic differences evident in the lower body and lungs These data indicate that imatinib mesylate protects mice from intranasal challenge by limiting spread of the virus from the site of initial infection to distal

Discussion �Actin tail polymerization and localization is analagous between the Vac. V, Var. V, and MPX �Usage of Abl or Src kinases by Vac. V, Var. V, and MPX are functionally redundant �Imatinib mesylate can be used in therapeutic context and is least immunosuppressive �Dasatinib and PD-166326 showed promise in vitro but where very

Pros and Cons Pros: Provide a novel idea and possible therapy by inhibiting viral release (inhibiting Abl family tyrosine kinases) Knocking-down cells images show very clear and convincing results of actin tail reduction � Cons: In cell experiments, all drug treatments are post infection. In animal experiments, some of the drugs are pre-treated. In animal experiments, the figures only show uninfected control (without drug treatments). Better have a control with only drug treatment. The drug treatment methods should be unified as i. n. (not i. p. ) Should unify the names of drugs (use only one nomenclature system) �

Thank You