Uklanjanje rastvorenih gasova iz vode i dezodorizacija vode
















- Slides: 19

Uklanjanje rastvorenih gasova iz vode i dezodorizacija vode ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

• izazivaju koroziju ili je njihovo prisustvo u vodi nepoželjno iz drugog razloga • najčešće ugljen dioksid, kiseonik i ponekad vodoniksulfid • degazacija vode uklanjanje rastvorenih gasova • fizičke metode (manja selektivnost, moguće je istovremeno ukloniti dva ili više gasova) • hemijske metode (specifične za svaki gas) ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Uklanjanje CO 2 • Slobodni ugljen-dioksid u prirodnim vodama se najvećim delom nalazi kao rastvoreni CO 2 gas i samo malim delom kao nedisosovana ugljena kiselina • vezani ugljen-dioksid se nalazi u obliku karbonata i bikarbonata • pripadajući ugljen-dioksid - količina slobodnog CO 2 koja je potrebna za održavanje bikarbonata u rastvoru • agresivni ugljen-dioksid - višak CO 2 od potrebne količine za stabilizaciju bikarbonata (pojava korozije metala) ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Postupci i uređaji za uklanjanje CO 2 iz vode • Mehanički postupci - višak slobodnog CO 2 iz vode se uklanja putem aeracije u degazatorima različitog tipa: • Tornjevi sa punjenjem za povećanje površine kontakta između vode i vazduha, • Barbotažne kolone, • Vakumski degazatori. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

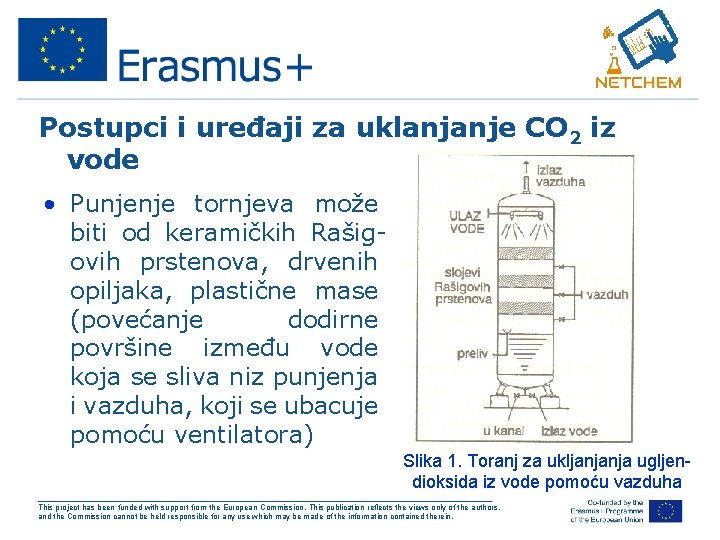
Postupci i uređaji za uklanjanje CO 2 iz vode • Punjenje tornjeva može biti od keramičkih Rašigovih prstenova, drvenih opiljaka, plastične mase (povećanje dodirne površine između vode koja se sliva niz punjenja i vazduha, koji se ubacuje pomoću ventilatora) Slika 1. Toranj za ukljanjanja ugljendioksida iz vode pomoću vazduha ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Postupci i uređaji za uklanjanje CO 2 iz vode • U barbotažnim kolonama se u vodu, koja se kreće niz kolonu, raspršuje vazduh pomoću mlaznice. • U vakuumskim degazatorima se pogodnom vacuum pumpom se otvaruje pritisak pod kojim voda ključa, usled čega se iz nje uklanja rastvoreni CO 2, ali I ostali rastvoreni gasovi, naročito kiseonik ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Postupci i uređaji za uklanjanje CO 2 iz vode • Hemijski postupci • prevođenje agresivnog CO 2 u vezani oblik filtracijom vode preko usitnjenog mermera (čist Ca. CO 3) uz formiranje kalcijumbikarbonata • nije pogodan za vode sa povećanim sadržajem gvožđa (zrnca mermernog peska se oblažu Fe(OH)3 i inakriviraju) • doziranje gašenog kreča kao krečne vode ili krečnog mleka. • Velika količina kreča može dovesti do pojave karbonatnih naslaga u cevovodima (može se upotrebiti i natrijumhidroksid ili natrijum-karbonat) • filtracija preko polužarenog magnezita (dolomita) ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Postupci i uređaji za uklanjanje CO 2 iz vode • filtracija preko polužarenog magnezita (dolomita) • žarenje na 6000 -7000 C magnezijum-karbonat prelazi u Mg. O, a kalcijum-karbonat ostaje nepromenjen • Voda se oslobađa viška CO 2 filtracijom preko ovakve mase, uz manje povećanje tvrdoće, nego prilikom filtracije preko mermernog peska ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Uklanjanje rastvorenog kiseonika • U toploj vodi izaziva koroziju • može se ukloniti: • Termičkim (deaeracija vode) i • Hemijskim postupcima (deoksigenacija vode) Termički postupci • na temperaturi ključanja rastvorljivost kiseonika, a i drugih gasova, jednaka nuli. • uklanjaju se i drugi rastvoreni gasovi, postupak se naziva deaeracija vode. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Uklanjanje rastvorenog kiseonika Hemijski postupci • dodatak jedinjenja redukcionog karaktera, koja se lako i brzo oksidišu rastvorenim kiseonikom i na taj način ga troše (uklanja se samo kiseonik – deoksigenacija) • natrijum-sulfat Na 2 SO 3 + O 2 → 2 Na 2 SO 4 • Na 2 SO 3 je jeftin, lako se priprema i dozira • Nedostatak - zaostaju rastvorene soli, a na visokim temperaturama može doći do hidrolize (formiranje SO 2, koji pevećava korozivnost pare i kondenzata) Na 2 SO 3 + H 2 O → 2 Na. OH + H 2 SO 3 → SO 2 + H 2 O ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Uklanjanje rastvorenog kiseonika • Hidrazin N 2 H 2 + O 2 → N 2 + 2 H 2 O • Reakcija teče brzo na T=1000 C i p. H=8. 7 • može da redukuje eventualno prisutne okside metala • Na temperaturi preko 1800 C termiči se raspada 3 N 2 H 2 → N 2 + 4 NH 3 2 N 2 H 2 → N 2 + H 2 + 2 NH 3 ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Uklanjanje H 2 S • Javlja se u nekim prirodnim vodama, u vodi za piće izaziva neprijatan miris • uklanja se oksidacijom sa hlorom H 2 S + Cl 2 → S + 2 HCl H 2 S + 4 Cl 2 + 4 H 2 O → H 2 SO 4 + 8 HCl ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Dezodorizacija vode • dezodoziranje vode – uklanjanje različitih supstanci organske ili neorganske prirode, ili mkroorganizama koji uslovljavaju pojavu neprijatnih, nesvojstvenih ukusa i mirisa • sumpor-vodonik, fenoli, različiti metabolički mikroorganizmi, hlor koji se koristi za dezinfekciju • adsorpcija na aktivnom uglju: • • Filtracija vode kroz sloj granulisanog aktivnog uglja, Dodatak sprašenog aktivnog uglja i filtracija. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Dezodorizacija vode - adsorpcija na aktivnom uglju • 0, 06 -0, 12 m 3 po m 3 dezodorisane vode • Nakon zasićenja sloja uglja adsorbovanim sastojcima treba obavljati njihovu desorbciju (provođenje vodene pare u obrnutom smeru) • Dalji postupci uklanjanja organoleptičkih aktivnih organskih suptstanci iz vode počivaju na oksidaciji sa hlorom, kalijum-permanganatom, ili ozonom. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

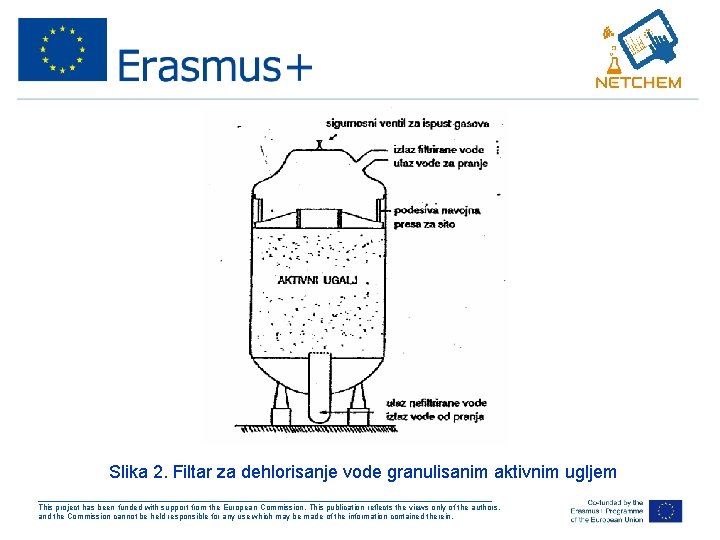
Dezodorizacija vode – dehlorisanje vode • filtracija vode kroz sloj granulisanog aktivnog uglja • voda se obično prevodi u smeru odozdo prema gore (slika 2) • Oksidacija ugljenika do ugljen-dioksida C + Cl 2 + 2 H 2 O → CO 2 + 4 HCl ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Slika 2. Filtar za dehlorisanje vode granulisanim aktivnim ugljem ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Dezodorizacija vode – dehlorisanje vode • Hemijski postupak - redukcija sa hlorida Na 2 S 2 O 3 do Na 2 S 2 O 3 + 4 Cl 2 + 5 H 2 O → 2 H 2 SO 4 +2 Na. Cl + 6 HCl ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Author, Editor and Referee References This remote access laboratory was created thanks to work done primarily at University of Niš. Contributors to this material were: ________ Refereeing of this material was done by: ___________ Editing into NETCHEM Format and onto NETCHEM platform was completed by: __________________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, ___________________________________________________ andhas the been Commission be held responsible for any use which may made of the information contained This project funded cannot with support from the European Commission. Thisbe publication reflects the views only of therein. the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

References and Supplemental Material The NETCHEM platform was established at the University of Nis in 2016 -2019 through the Erasmus Programme. Please contact a NETCHEM representatives at your institution or visit our website for an expanded contact list. The work included had been led by the NETCHEM staff at your institution. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, ___________________________________________________ andhas the been Commission be held responsible for any use which may made of the information contained This project funded cannot with support from the European Commission. Thisbe publication reflects the views only of therein. the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.
 Raspodela molekula gasa po brzinama
Raspodela molekula gasa po brzinama Disperzni sistem
Disperzni sistem Ispitivanje lambda sonde
Ispitivanje lambda sonde Inertni argon gas
Inertni argon gas Koncentracija molekula gasa
Koncentracija molekula gasa Sistem organa za disanje kod coveka
Sistem organa za disanje kod coveka Kontrola izduvnih gasova
Kontrola izduvnih gasova Mie vode moll
Mie vode moll Gustina morske vode
Gustina morske vode Plava sviecka vo vode
Plava sviecka vo vode Hipotermalne vode
Hipotermalne vode Izvore vode žive
Izvore vode žive živočíchy žijúce pri vode
živočíchy žijúce pri vode žive morske mijene
žive morske mijene Ptica selica zivi u vodi i pored vode
Ptica selica zivi u vodi i pored vode Celinske vode
Celinske vode Dipolarnost vode
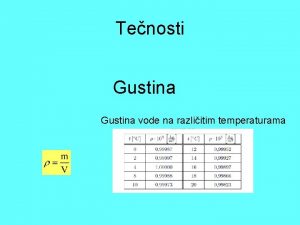
Dipolarnost vode Gustina vode na razlicitim temperaturama
Gustina vode na razlicitim temperaturama Površina obalnog mora
Površina obalnog mora Hajducke vode skijanje
Hajducke vode skijanje