Total Synthesis of Manzamine A and Related Alkaloids



- Slides: 17

Total Synthesis of Manzamine A and Related Alkaloids Pavol Jakubec, Alison Hawkins, Wolfgang Felzmann, and Darren J. Dixon* J. Am. Chem. Soc. , 2012, 134, 17482– 17485 1 Speaker: 王湘閔

Outline �Motivation �Introduction �Past work �Retrosynthesis �Synthesis �Conclusion �Summary 2

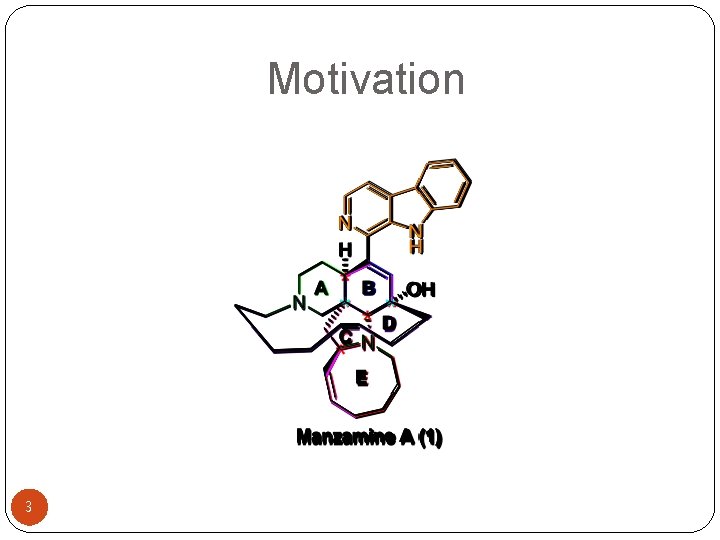
Motivation 3

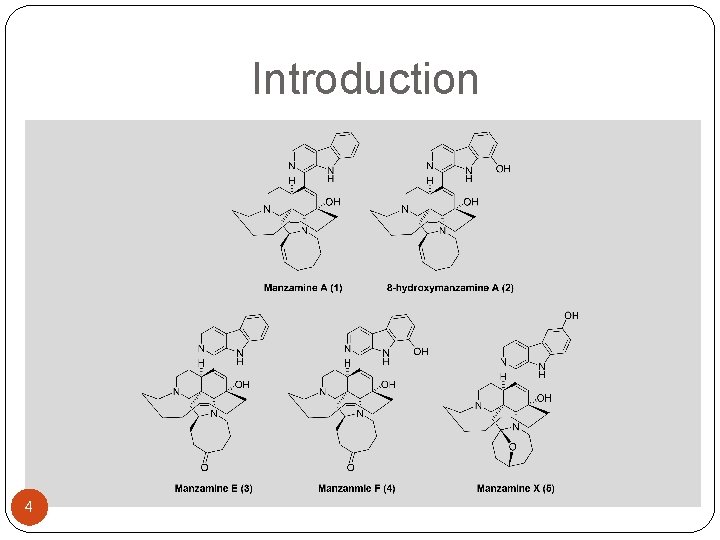
Introduction �Isolation: Alkaloids were first isolated from the marine sponge Haliclona sp. in the Okinawa Sea by Higa et al. in 1986. �Biological activities: insecticidal, cytotoxicity, antibacterial, anti-HIV-I, anti-infective, anti. Alzheimer diseases, and anti-malarial activity. �P 388 mouse leukaemia cells (IC 50 = 0. 07 µg/m. L or 2. 4 µg/m. L) 4

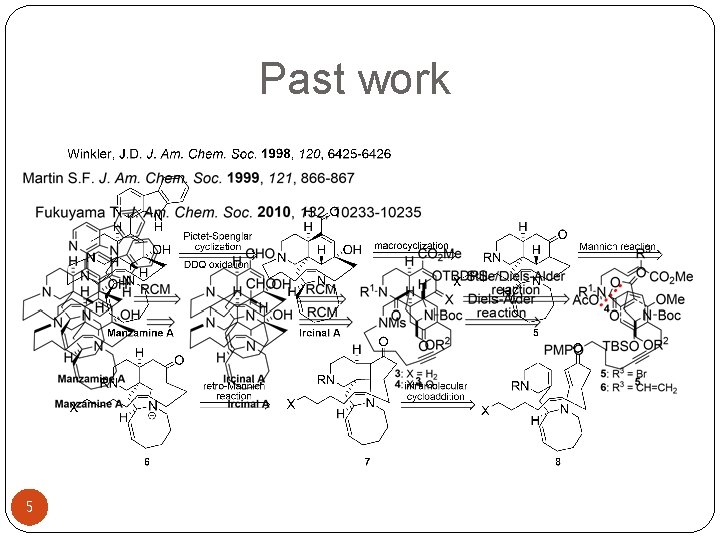
Past work 5

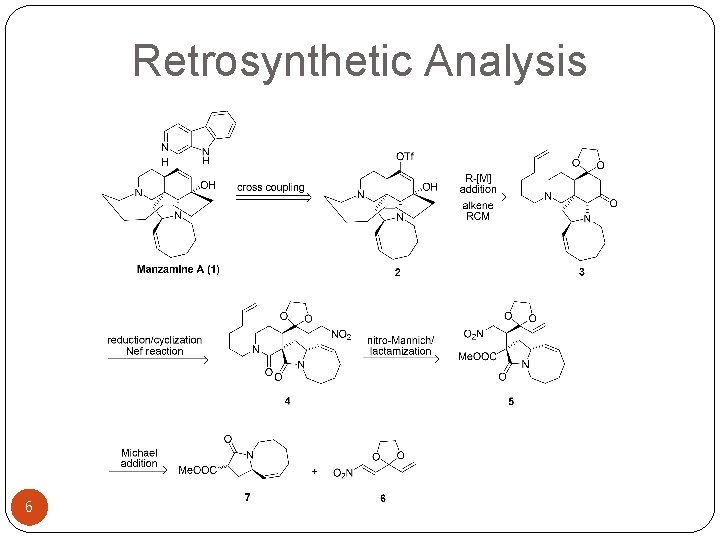
Retrosynthetic Analysis 6

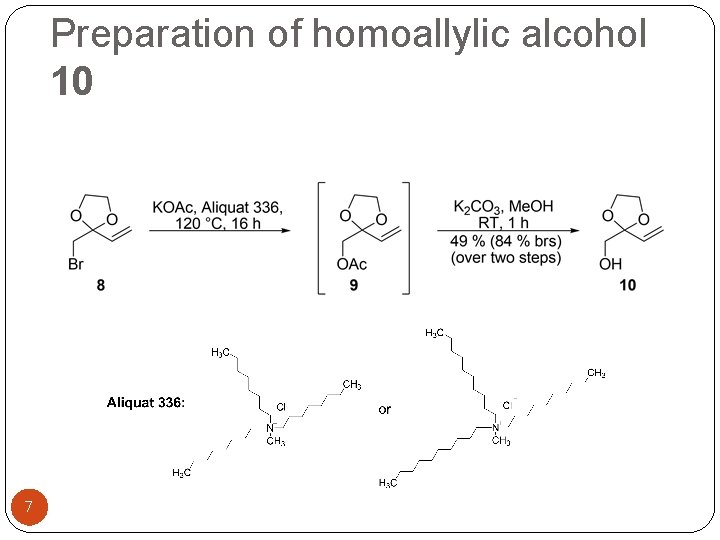
Preparation of homoallylic alcohol 10 7

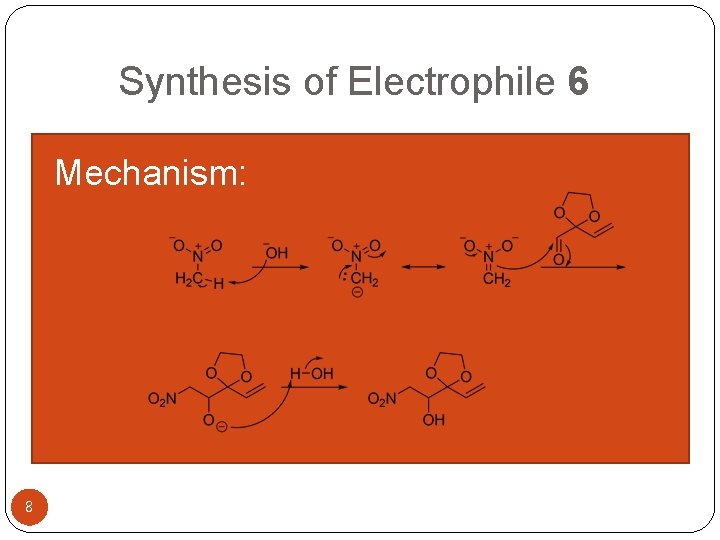
Synthesis of Electrophile 6 Mechanism: 8

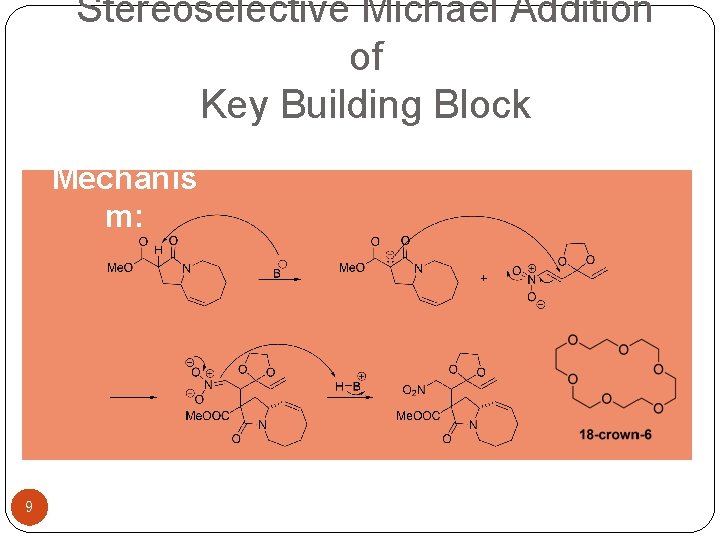
Stereoselective Michael Addition of Key Building Block Mechanis m: 9

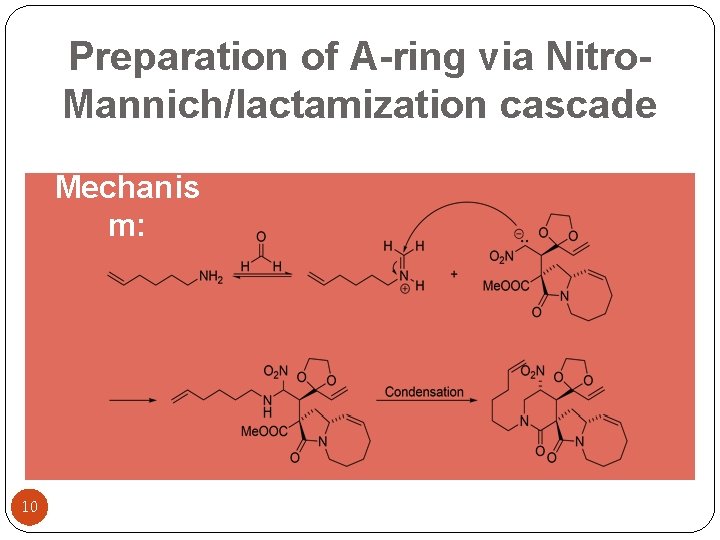
Preparation of A-ring via Nitro. Mannich/lactamization cascade Mechanis m: 10

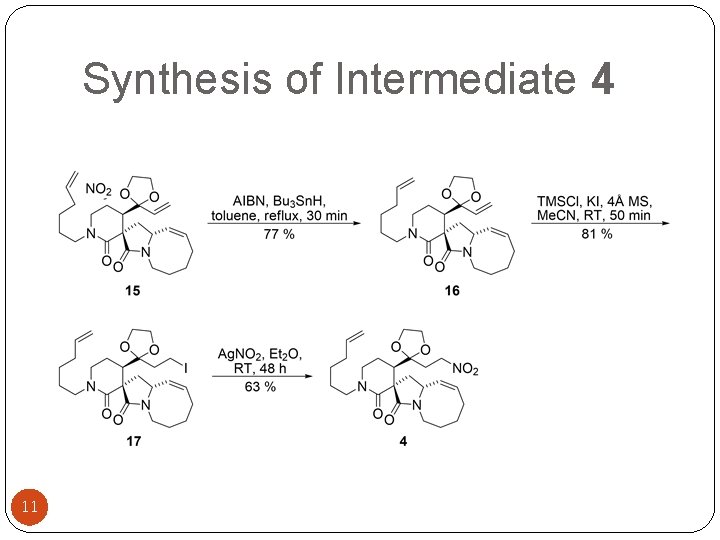
Synthesis of Intermediate 4 11

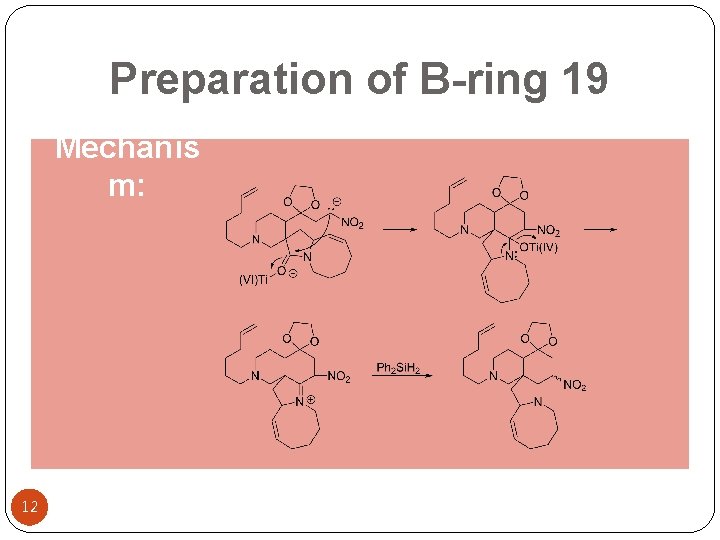
Preparation of B-ring 19 Mechanis m: 12

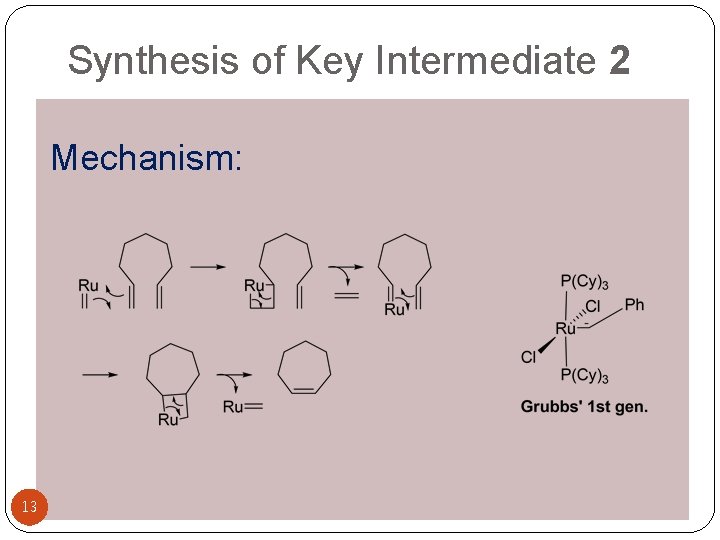
Synthesis of Key Intermediate 2 Mechanism of Nef Reaction Mechanism: 13

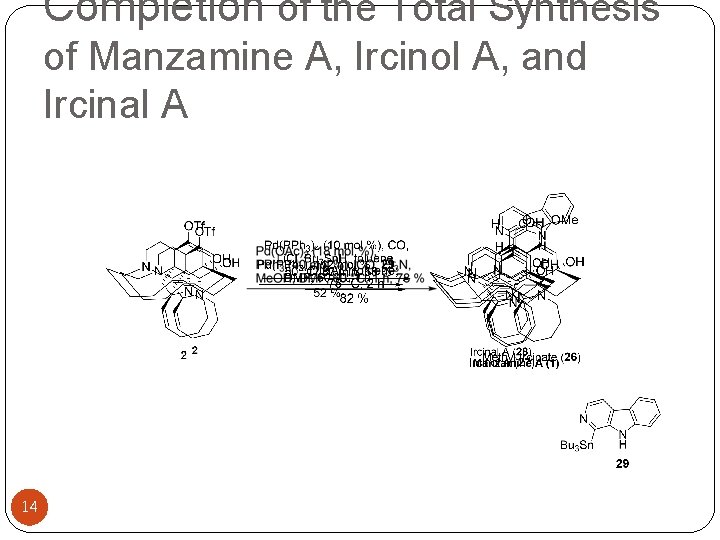
Completion of the Total Synthesis of Manzamine A, Ircinol A, and Ircinal A 14

Conclusion �Author and co-workers have developed a short and stereoselective synthesis of manzamine A (1) via 18 steps, total yield 1. 03 %. �Enol triflate 2 is a synthetically valuable late-stage intermediate. �Key steps: Stereoselective Michael addition Nitro-Mannich/Lactamization cascade Z-selective alkene RCM 15

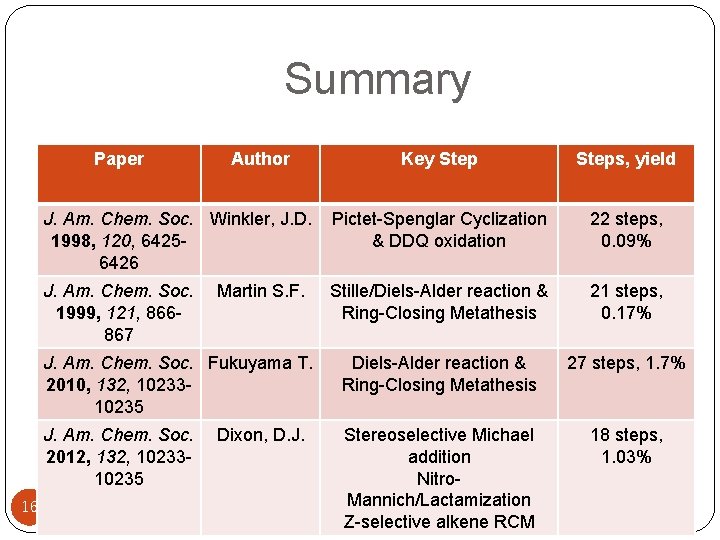
Summary Paper 16 Author Key Steps, yield J. Am. Chem. Soc. Winkler, J. D. 1998, 120, 64256426 Pictet-Spenglar Cyclization & DDQ oxidation 22 steps, 0. 09% J. Am. Chem. Soc. 1999, 121, 866867 Stille/Diels-Alder reaction & Ring-Closing Metathesis 21 steps, 0. 17% J. Am. Chem. Soc. Fukuyama T. 2010, 132, 1023310235 Diels-Alder reaction & Ring-Closing Metathesis 27 steps, 1. 7% J. Am. Chem. Soc. 2012, 132, 1023310235 Stereoselective Michael addition Nitro. Mannich/Lactamization Z-selective alkene RCM 18 steps, 1. 03% Martin S. F. Dixon, D. J.

17
 Two type of physical fitness
Two type of physical fitness Skill related fitness vs health related fitness
Skill related fitness vs health related fitness Example of synthesis in research
Example of synthesis in research Pyridine piperidine alkaloids
Pyridine piperidine alkaloids Alkaloids definition pharmacognosy
Alkaloids definition pharmacognosy Norlupinane
Norlupinane Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Ergoline alkaloids
Ergoline alkaloids Alkaloid examples
Alkaloid examples Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Alkaloids derived from tyrosine
Alkaloids derived from tyrosine Purine alkaloids
Purine alkaloids Purine alkaloids
Purine alkaloids Peomus
Peomus Purine alkaloids
Purine alkaloids Belladonna alkaloids drugs
Belladonna alkaloids drugs Tropane alkaloids
Tropane alkaloids Ergot alkaloids alpha blockers
Ergot alkaloids alpha blockers





























