THE UNIVERSITY OF THE STATE OF NEW YORK






















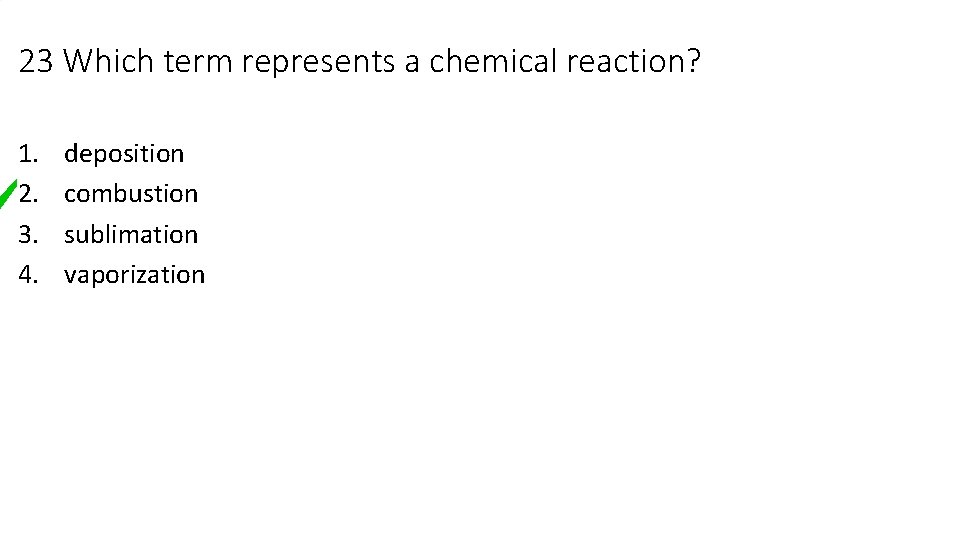











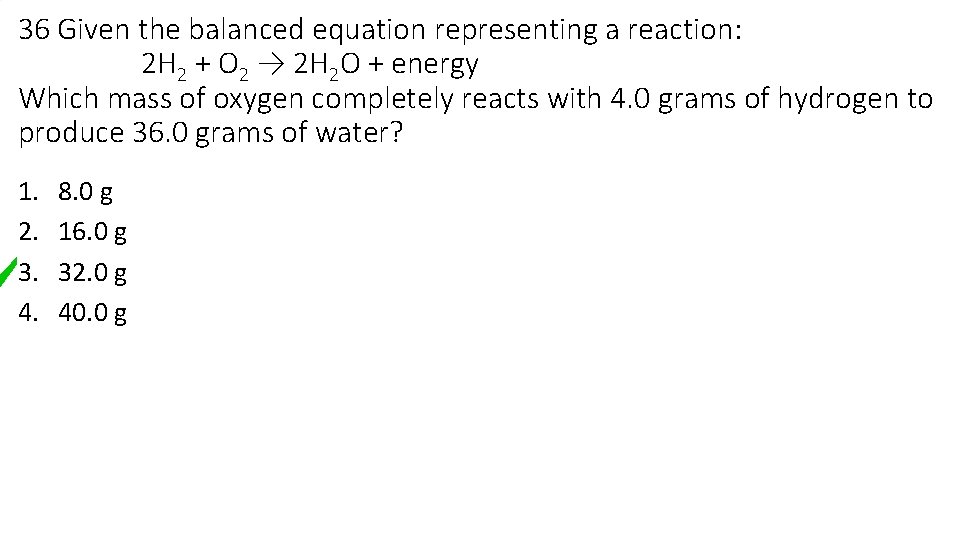
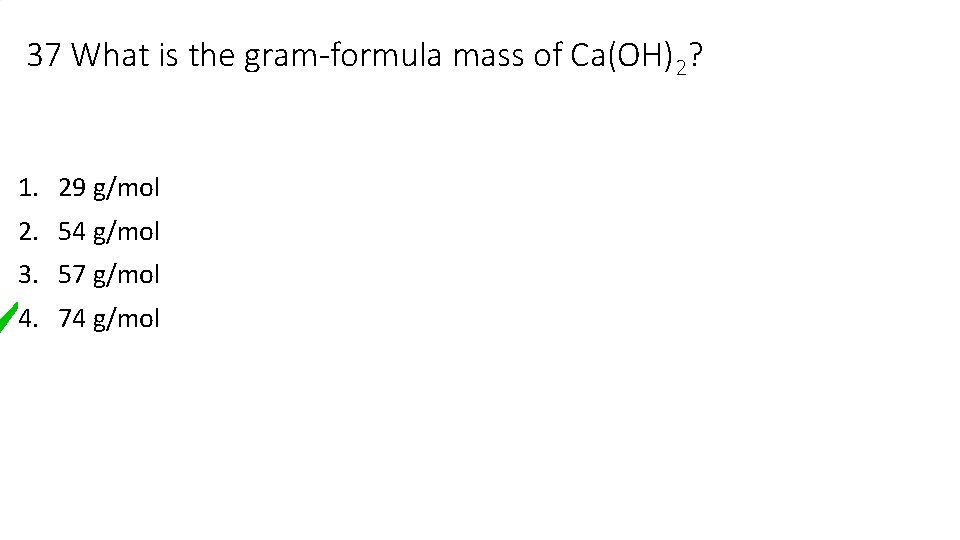





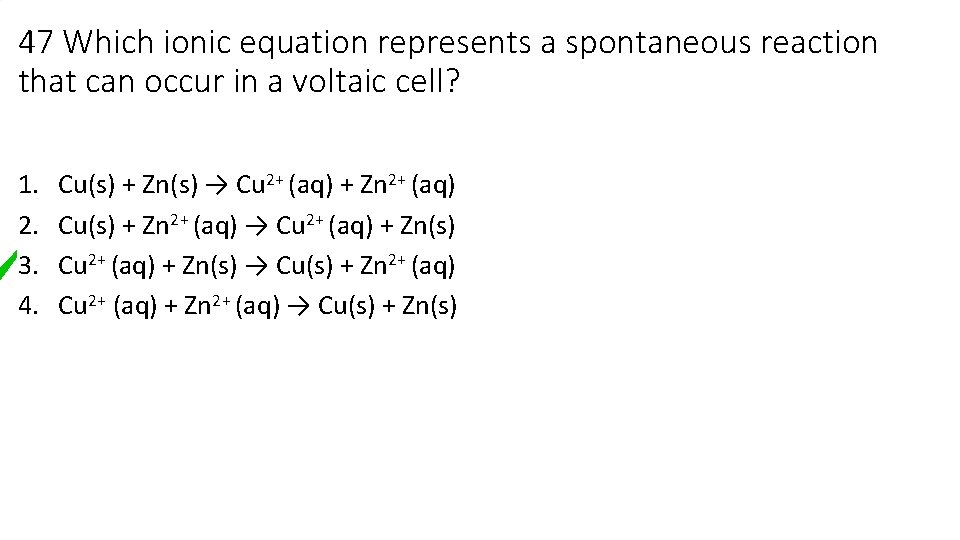
- Slides: 51

THE UNIVERSITY OF THE STATE OF NEW YORK REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING CHEMISTRY January 25, 2017

1 Which statement describes the location of two types of subatomic particles in a helium atom? 1. Protons and neutrons are located in the nucleus. 2. Protons and neutrons are located outside the nucleus. 3. Protons and electrons are located in the nucleus. 4. Protons and electrons are located outside the nucleus.

2 An atom that contains six protons, six neutrons, and six electrons has a mass of approximately 1. 2. 3. 4. 12 u 12 g 18 u 18 g

3 Which term identifies the most probable location of an electron in the wave-mechanical model of the atom? 1. 2. 3. 4. anode orbital nucleus cathode

4 Which element has atoms in the ground state with the greatest number of valence electrons? 1. 2. 3. 4. Tin Sulfur Arsenic Flourine

5 Which group on the Periodic Table has two elements that exist as gases at STP? 1. 2. 3. 4. Group 1 Group 2 Group 16 Group 17

6 Which list of elements contains a metal, a metalloid, and a nonmetal? 1. 2. 3. 4. Ag, Si, I 2 Ge, As, Ne K, Cu, Br 2 S, Cl 2, Ar

7 What is the charge of the nucleus of a copper atom? 1. 2. 3. 4. +1 +2 +29 +64

8 At STP, O 2(g) and O 3(g) have different properties because O 3(g) has 1. more dense nuclei than in O 2(g) 2. more protons per atom than in O 2(g) 3. molecules with a different structure than in O 2(g) 4. molecules with fewer covalent bonds than in O 2(g)

9 A compound is a substance composed of two or more elements that are 1. physically mixed in a fixed proportion 2. physically mixed in a variable proportion 3. chemically combined in a fixed proportion 4. chemically combined in a variable proportion

10 Which element has the highest boiling point at standard pressure? 1. 2. 3. 4. Mg Na Rb Sr

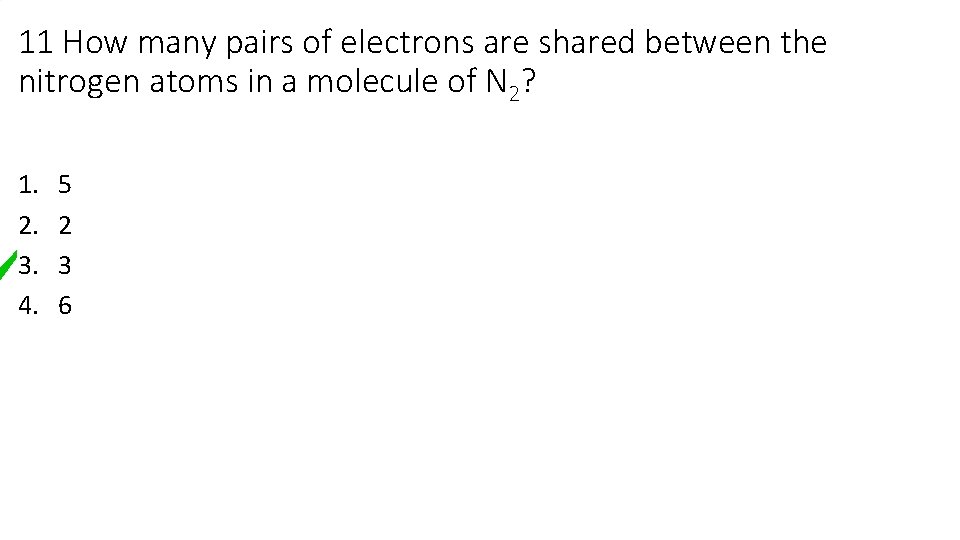
11 How many pairs of electrons are shared between the nitrogen atoms in a molecule of N 2? 1. 2. 3. 4. 5 2 3 6

12 A molecule must be nonpolar if the molecule 1. is linear 2. is neutral 3. has ionic and covalent bonding 4. has a symmetrical charge distribution

13 Which property is used to determine the degree of polarity between two bonded atoms? 1. 2. 3. 4. density electronegativity pressure temperature

14 In a chemical reaction, a catalyst provides an alternate reaction pathway that 1. decreases the concentration of the products 2. increases the concentration of the reactants 3. has a lower activation energy 4. has a higher activation energy

15 Which substance can be decomposed by chemical means? 1. 2. 3. 4. cobalt krypton methane zirconium

16 Which sample of matter represents a mixture? 1. 2. 3. 4. aqueous ammonia gaseous ethane liquid mercury solid iodine

17 Differences in which property allow the separation of a sample of sand seawater by filtration? 1. 2. 3. 4. concentration of ions volume of sample mass of sample particle size

18 Which process is a chemical change? 1. 2. 3. 4. evaporating an alcohol subliming of iodine melting an ice cube rusting of iron

19 Which term represents an intermolecular force in a sample of water? 1. 2. 3. 4. hydrogen bonding covalent bonding metallic bonding ionic bonding

20 Which sample of matter has particles arranged in a crystalline structure? 1. 2. 3. 4. Ne(g) Br 2(ℓ) Na. Cl(aq) Cu. SO 4(s)

21 Which term is defined as a measure of the randomness of a system? 1. 2. 3. 4. heat entropy pressure temperature

22 Which formula represents an alkane? 1. 2. 3. 4. C 2 H 2 C 2 H 4 C 3 H 4 C 3 H 8
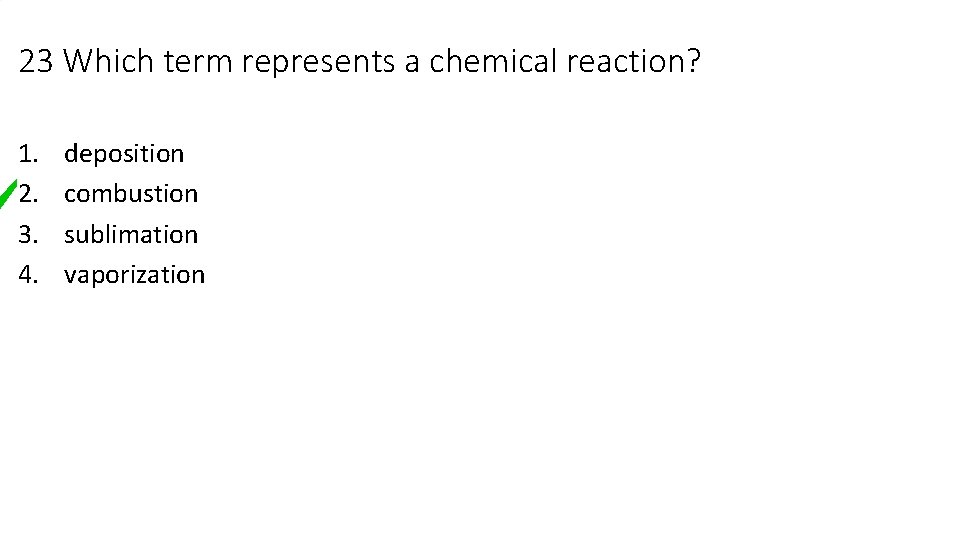
23 Which term represents a chemical reaction? 1. 2. 3. 4. deposition combustion sublimation vaporization

24 Which type of reaction includes esterification and polymerization? 1. decomposition 2. neutralization 3. organic 4. nuclear

25 In a redox reaction, the total number of electrons lost is 1. less than the total number of electrons gained 2. greater than the total number of electrons gained 3. equal to the total number of electrons gained 4. unrelated to the total number of electrons gained

26 Which type of equation can represent the oxidation occurring in a reaction? 1. a double-replacement reaction equation 2. a half-reaction equation 3. a neutralization reaction equation 4. a transmutation reaction equation

27 The electrical conductivity of an aqueous solution depends on the concentration of which particles in the solution? 1. 2. 3. 4. molecules electrons atoms ions

28 An electrolytic cell differs from a voltaic cell because an electrolytic cell 1. generates its own energy from a spontaneous physical reaction 2. generates its own energy from a nonspontaneous physical reaction 3. requires an outside energy source for a spontaneous chemical reaction to occur 4. requires an outside energy source for a nonspontaneous chemical reaction to occur

29 A sample of which radioisotope emits particles having the greatest mass? 1. 137 Cs 2. 53 Fe 3. 220 Fr 4. 3 H

30 Which term represents a nuclear reaction? 1. 2. 3. 4. combustion fermentation transmutation saponification

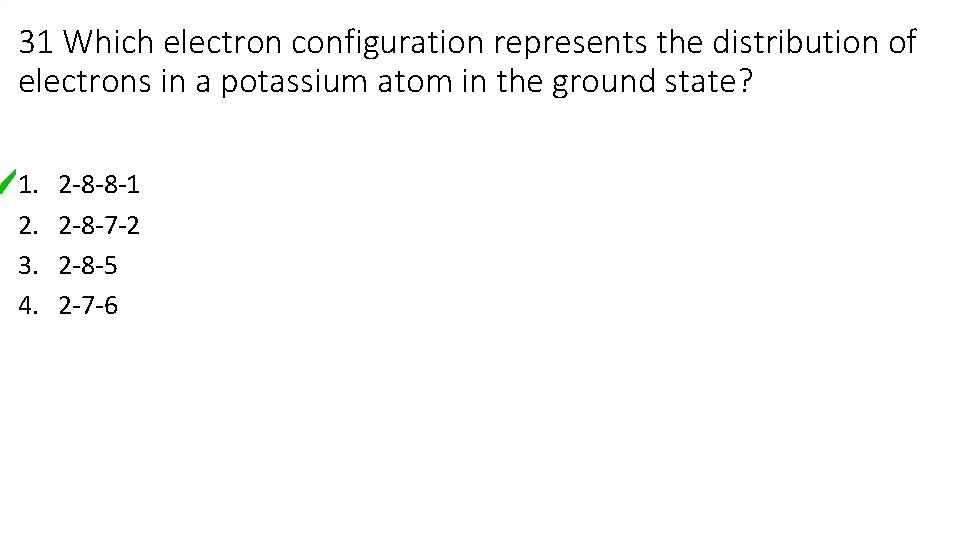
31 Which electron configuration represents the distribution of electrons in a potassium atom in the ground state? 1. 2. 3. 4. 2 -8 -8 -1 2 -8 -7 -2 2 -8 -5 2 -7 -6

32 At STP, which element is malleable and a good conductor of electricity? 1. 2. 3. 4. xenon silicon platinum hydrogen

33 Which general trends in atomic radius and electronegativity are observed as the elements in Period 3 are considered in order of increasing atomic number? 1. Atomic radius decreases and electronegativity increases. 2. Atomic radius increases and electronegativity decreases. 3. Both atomic radius and electronegativity increase. 4. Both atomic radius and electronegativity decrease.

34 What is the chemical name for Na 2 SO 3? 1. 2. 3. 4. sodium sulfite sodium sulfate sodium sulfide sodium thiosulfate

35 Which molecular formula is also an empirical formula? 1. 2. 3. 4. C 6 H 6 H 2 O 2 N 2 H 4 N 2 O 5
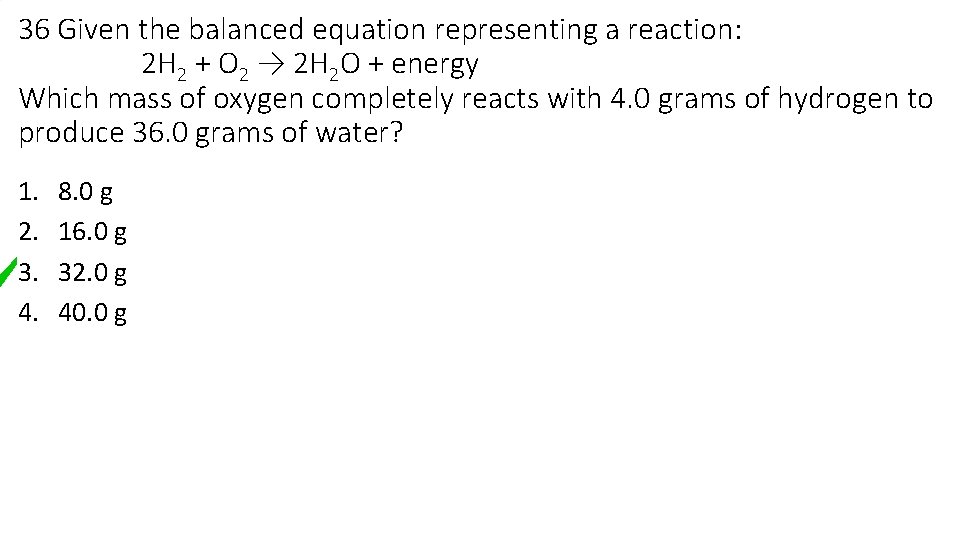
36 Given the balanced equation representing a reaction: 2 H 2 + O 2 → 2 H 2 O + energy Which mass of oxygen completely reacts with 4. 0 grams of hydrogen to produce 36. 0 grams of water? 1. 2. 3. 4. 8. 0 g 16. 0 g 32. 0 g 40. 0 g
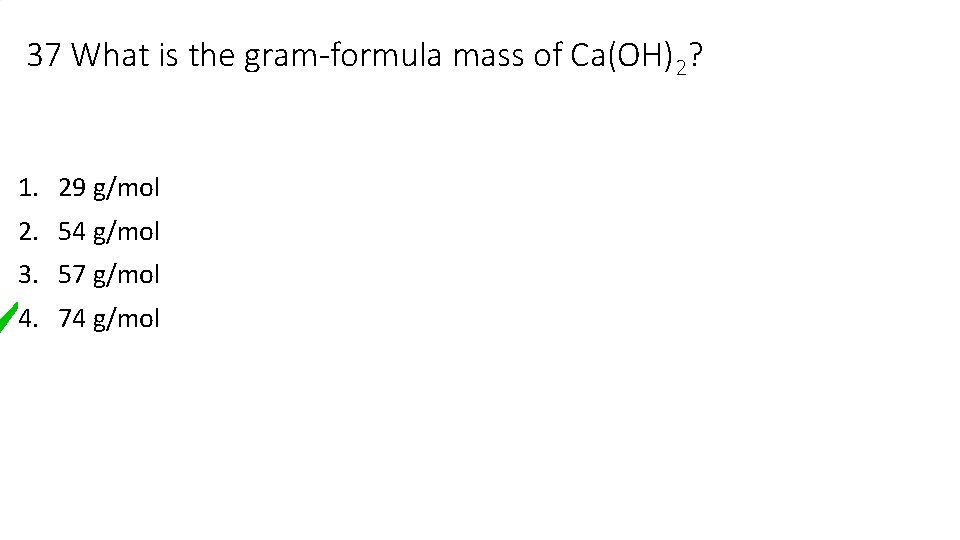
37 What is the gram-formula mass of Ca(OH)2? 1. 29 g/mol 2. 54 g/mol 3. 57 g/mol 4. 74 g/mol

38 Given the equation representing a reaction: H 2(g) + I 2(g) → 2 HI(g) Which statement describes the energy changes that occur in this reaction? 1. Energy is absorbed as bonds are formed, only. 2. Energy is released as bonds are broken, only. 3. Energy is absorbed as bonds are formed, and energy is released as bonds are broken. 4. Energy is absorbed as bonds are broken, and energy is released as bonds are formed.

39 Based on Table F, which compound is least soluble in water? •

40 How many joules of heat are absorbed to raise the temperature of 435 grams of water at 1 atm from 25°C to its boiling point, 100. °C? 1. 2. 3. 4. 5 x 104 J 1. 4 x 105 J 2. 5 x 107 J 7. 4 x 107 J
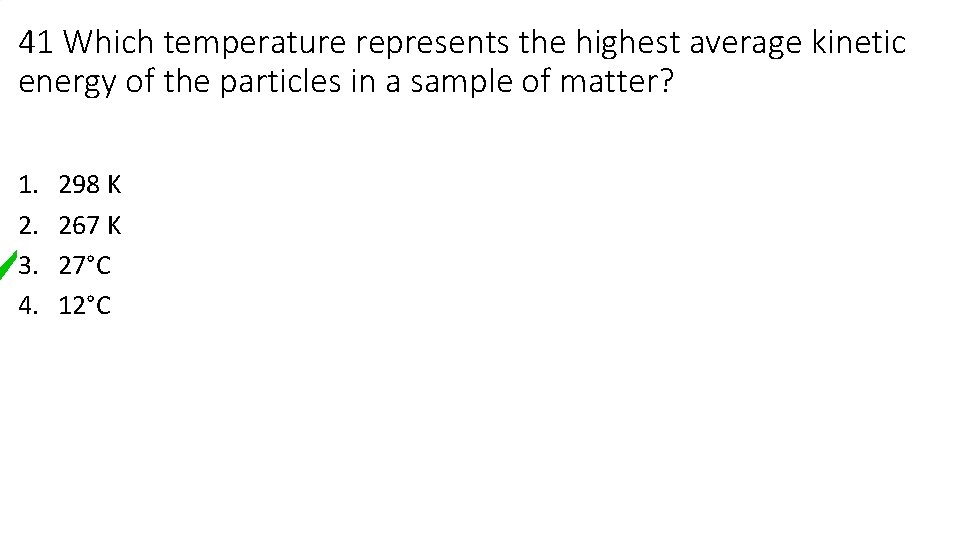
41 Which temperature represents the highest average kinetic energy of the particles in a sample of matter? 1. 2. 3. 4. 298 K 267 K 27°C 12°C

42 Which change in the H+ ion concentration of an aqueous solution represents a decrease of one unit on the p. H scale? 1. 2. 3. 4. a tenfold increase a tenfold decrease a hundredfold increase a hundredfold decrease

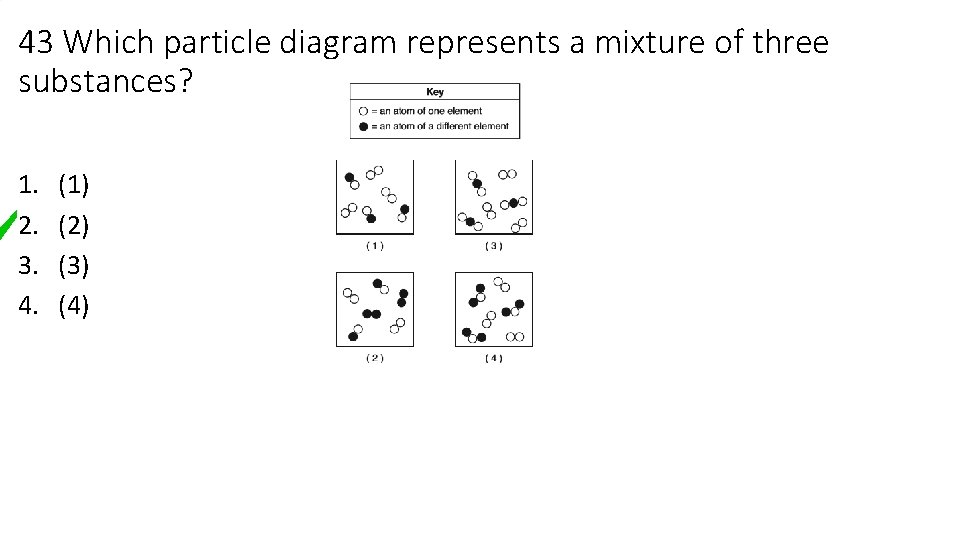
43 Which particle diagram represents a mixture of three substances? 1. 2. 3. 4. (1) (2) (3) (4)

44 Given the equation representing a system at equilibrium: PCl 5(g) — PCl 3(g) + Cl 2(g) Which statement describes this system? 1. The concentration of PCl 5(g) is increasing. 2. The concentration of PCl 5(g) is decreasing. 3. The concentrations of PCl 5(g) and PCl 3(g) are equal. 4. The concentrations of PCl 5(g) and PCl 3(g) are constant.

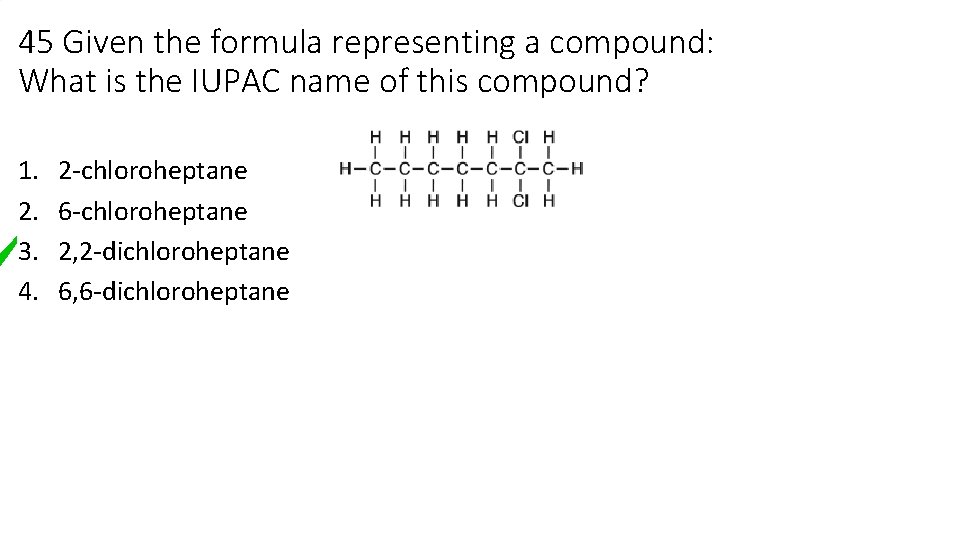
45 Given the formula representing a compound: What is the IUPAC name of this compound? 1. 2. 3. 4. 2 -chloroheptane 6 -chloroheptane 2, 2 -dichloroheptane 6, 6 -dichloroheptane

46 Given the equation representing a reaction: Sn 4+ (aq) + 2 e- → Sn 2+ (aq) Which term best describes this reaction? 1. 2. 3. 4. ionization neutralization oxidation reduction
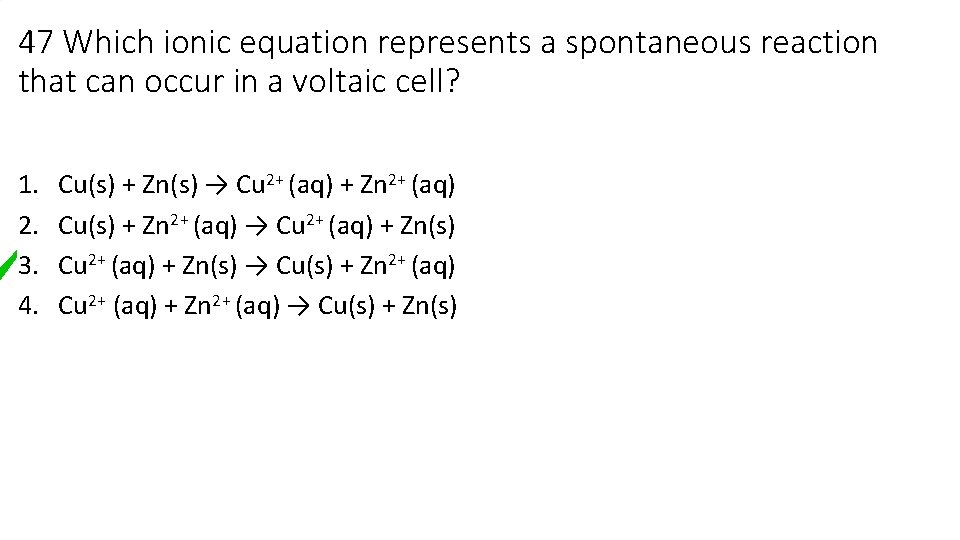
47 Which ionic equation represents a spontaneous reaction that can occur in a voltaic cell? 1. 2. 3. 4. Cu(s) + Zn(s) → Cu 2+ (aq) + Zn 2+ (aq) Cu(s) + Zn 2+ (aq) → Cu 2+ (aq) + Zn(s) → Cu(s) + Zn 2+ (aq) Cu 2+ (aq) + Zn 2+ (aq) → Cu(s) + Zn(s)

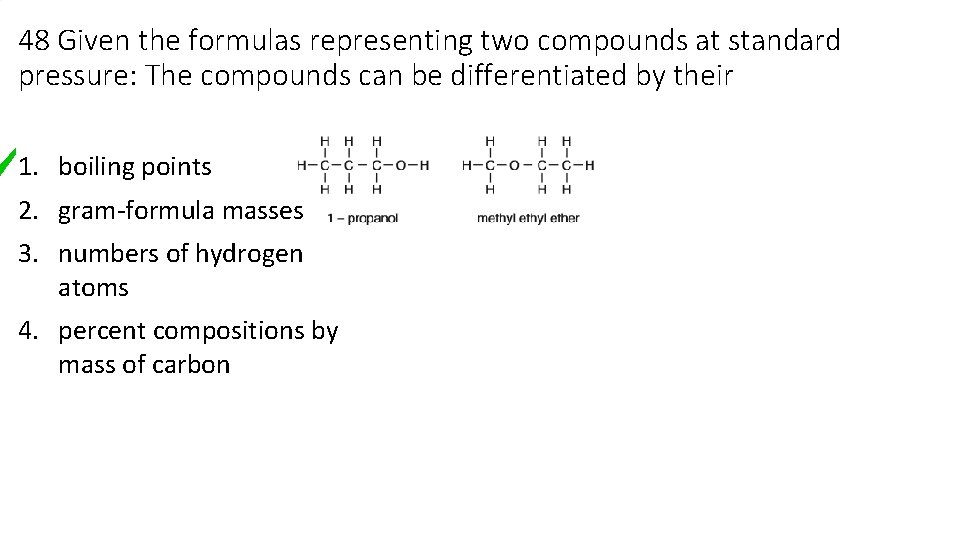
48 Given the formulas representing two compounds at standard pressure: The compounds can be differentiated by their 1. boiling points 2. gram-formula masses 3. numbers of hydrogen atoms 4. percent compositions by mass of carbon

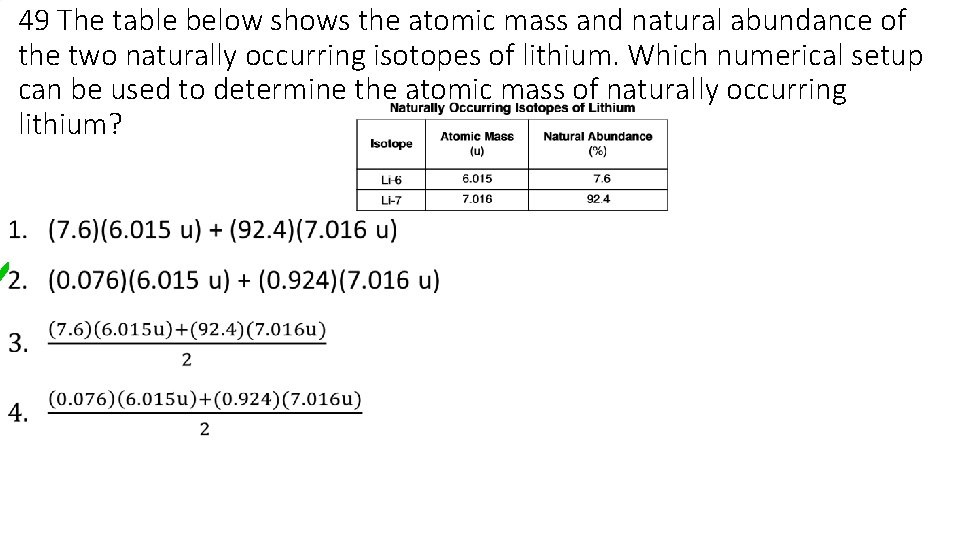
49 The table below shows the atomic mass and natural abundance of the two naturally occurring isotopes of lithium. Which numerical setup can be used to determine the atomic mass of naturally occurring lithium? •

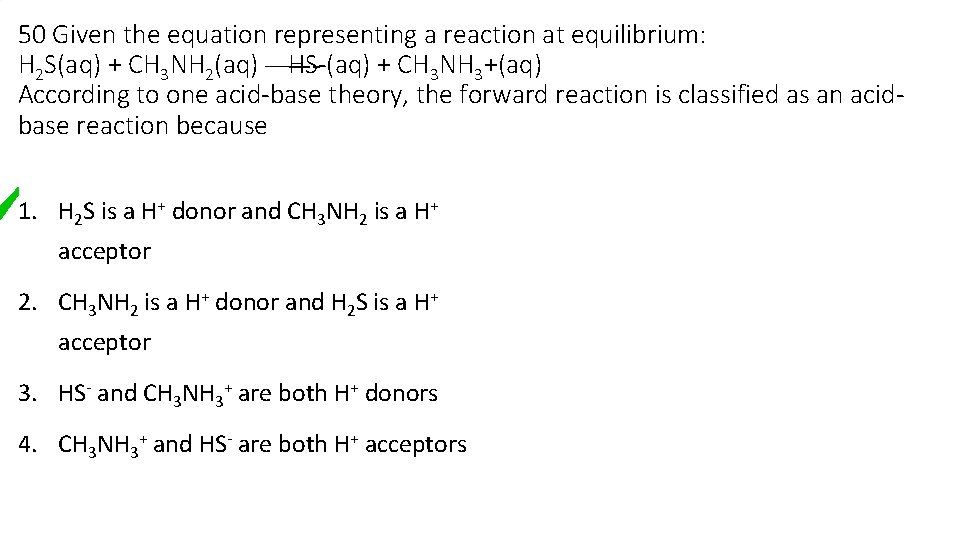
50 Given the equation representing a reaction at equilibrium: H 2 S(aq) + CH 3 NH 2(aq) — HS-(aq) + CH 3 NH 3+(aq) According to one acid-base theory, the forward reaction is classified as an acidbase reaction because 1. H 2 S is a H+ donor and CH 3 NH 2 is a H+ acceptor 2. CH 3 NH 2 is a H+ donor and H 2 S is a H+ acceptor 3. HS- and CH 3 NH 3+ are both H+ donors 4. CH 3 NH 3+ and HS- are both H+ acceptors
 Nurse practitioner association of new york state
Nurse practitioner association of new york state Ny state tap
Ny state tap New york state teacher certification examinations
New york state teacher certification examinations New york state science standards
New york state science standards State test 2018
State test 2018 New york state mesonet
New york state mesonet Ny state vegetable
Ny state vegetable Gigp-28
Gigp-28 New york state professional firefighters association
New york state professional firefighters association Ny immunization registry
Ny immunization registry New york state association of transportation engineers
New york state association of transportation engineers Nysid catalog
Nysid catalog Department of criminal justice services ny
Department of criminal justice services ny Nysrc
Nysrc Nysdot hdm chapter 5
Nysdot hdm chapter 5 What is nytprin
What is nytprin Rn scope of practice ny
Rn scope of practice ny New york state fish
New york state fish New york state nickname
New york state nickname New york state medicaid prior authorization
New york state medicaid prior authorization New york state amateur hockey association
New york state amateur hockey association New york state county highway superintendents association
New york state county highway superintendents association New york pennsylvania new jersey delaware
New york pennsylvania new jersey delaware Marquee cinema - new hartford, ny
Marquee cinema - new hartford, ny Both new hampshire and new york desire more territory
Both new hampshire and new york desire more territory Both new hampshire and new york desire more territory
Both new hampshire and new york desire more territory York university bad reputation
York university bad reputation Moodle performance tuning
Moodle performance tuning York university computer science
York university computer science Richard anderson york university
Richard anderson york university Brenton diaz york university
Brenton diaz york university Yorku myfile
Yorku myfile Jeff edmonds 3101
Jeff edmonds 3101 David phipps york university
David phipps york university Gibbs reflective cycle social work
Gibbs reflective cycle social work York university turnitin
York university turnitin Rem module york
Rem module york York university myanmar
York university myanmar Business etiquette in new york
Business etiquette in new york Procedural vs substantive due process
Procedural vs substantive due process Merriweather library buffalo new york
Merriweather library buffalo new york East and west egg in the great gatsby
East and west egg in the great gatsby 17 squared
17 squared Taoki new york
Taoki new york Map of new york and philadelphia
Map of new york and philadelphia Volomandra
Volomandra Nyctcm
Nyctcm Upfront new york times
Upfront new york times Ccta new york
Ccta new york Lega amerike
Lega amerike Nyhre
Nyhre Zone di new york
Zone di new york