The RESPOND Study at OneYear Primary Endpoint Outcomes


- Slides: 15

The RESPOND Study at One-Year: Primary Endpoint Outcomes with a Repositionable and Fully Retrievable Aortic Valve in Routine Clinical Practice Nicolas M. Van Mieghem, MD, Ph. D Thoraxcenter, Erasmus MC, Rotterdam, the Netherlands Jochen Wöhrle, MD; David Hildick-Smith, MD; Sabine Bleiziffer, MD; Daniel J Blackman, MD; Mohamed Abdel-Wahab, MD; Ulrich Gerckens, MD; Axel Linke, MD, Ph. D; Hüseyin Ince, MD, Ph. D; Peter Wenaweser, MD; Dominic J. Allocco, MD; Keith D. Dawkins, MD; Volkmar Falk, MD, Ph. D on behalf of the RESPOND Investigators

Speaker's name: Nicolas M Van Mieghem, MD, Ph. D I have the following potential conflicts of interest to report: • Institutional grant/research support: Abbott, Boston Scientific, Claret, Medtronic, Pulse. Cath BV The RESPOND study is sponsored and funded by Boston Scientific Corporation.

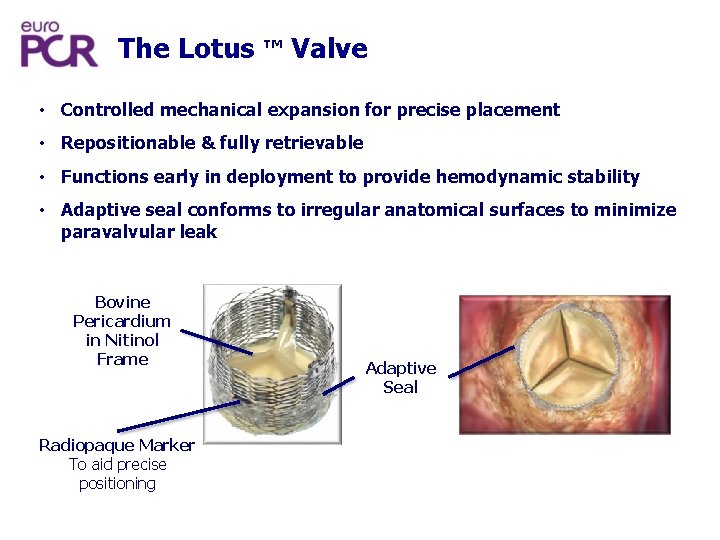
The Lotus ™ Valve • Controlled mechanical expansion for precise placement • Repositionable & fully retrievable • Functions early in deployment to provide hemodynamic stability • Adaptive seal conforms to irregular anatomical surfaces to minimize paravalvular leak Bovine Pericardium in Nitinol Frame Radiopaque Marker To aid precise positioning Adaptive Seal

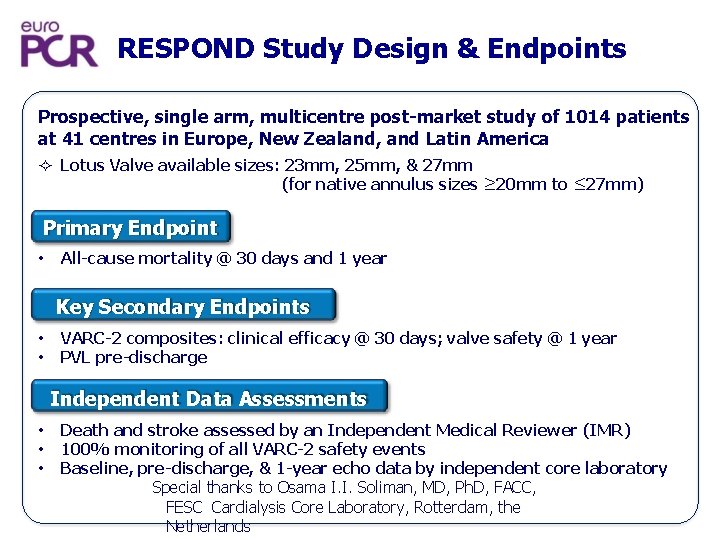
RESPOND Study Design & Endpoints Prospective, single arm, multicentre post-market study of 1014 patients at 41 centres in Europe, New Zealand, and Latin America Lotus Valve available sizes: 23 mm, 25 mm, & 27 mm (for native annulus sizes ≥ 20 mm to ≤ 27 mm) Primary Endpoint • All-cause mortality @ 30 days and 1 year Key Secondary Endpoints • • VARC-2 composites: clinical efficacy @ 30 days; valve safety @ 1 year PVL pre-discharge Independent Data Assessments • • • Death and stroke assessed by an Independent Medical Reviewer (IMR) 100% monitoring of all VARC-2 safety events Baseline, pre-discharge, & 1 -year echo data by independent core laboratory Special thanks to Osama I. I. Soliman, MD, Ph. D, FACC, FESC Cardialysis Core Laboratory, Rotterdam, the Netherlands

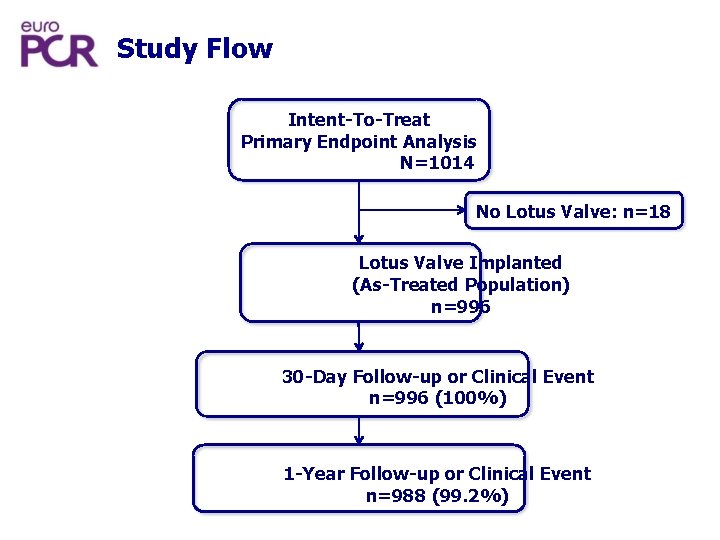
Study Flow Intent-To-Treat Primary Endpoint Analysis N=1014 No Lotus Valve: n=18 Lotus Valve Implanted (As-Treated Population) n=996 30 -Day Follow-up or Clinical Event n=996 (100%) 1 -Year Follow-up or Clinical Event n=988 (99. 2%)

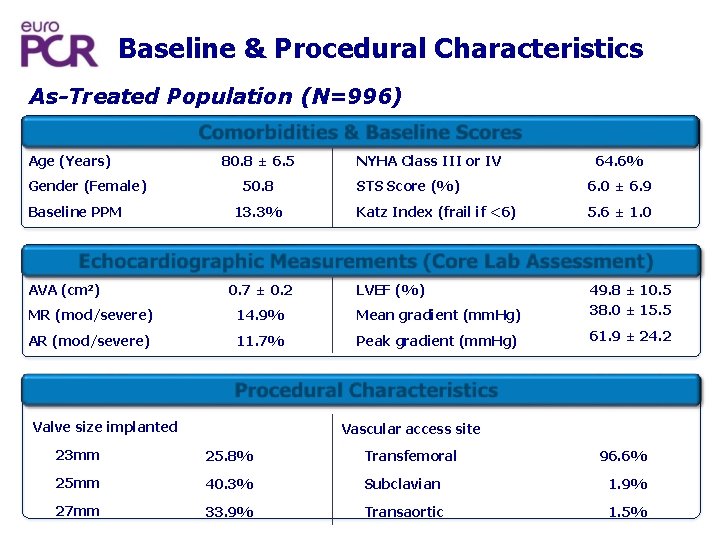
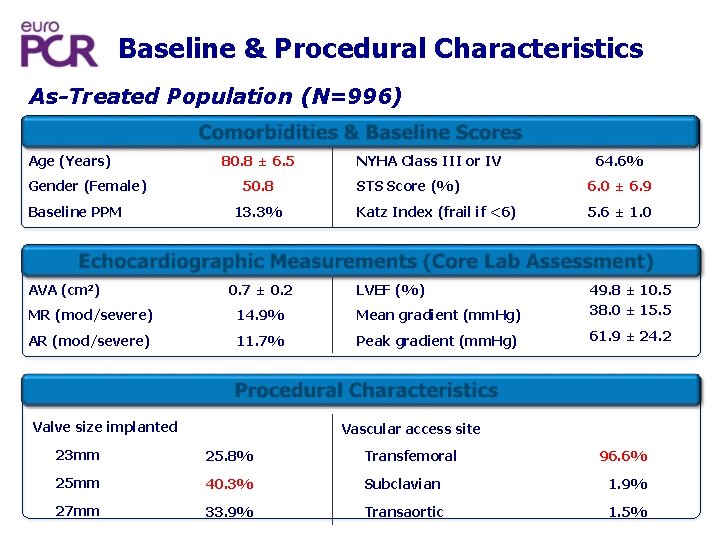
Baseline & Procedural Characteristics As-Treated Population (N=996) Comorbidities & Baseline Scores Age (Years) Gender (Female) Baseline PPM 80. 8 ± 6. 5 50. 8 13. 3% NYHA Class III or IV 64. 6% STS Score (%) 6. 0 ± 6. 9 Katz Index (frail if <6) 5. 6 ± 1. 0 Echocardiographic Measurements (Core Lab Assessment) AVA (cm 2) 0. 7 ± 0. 2 LVEF (%) MR (mod/severe) 14. 9% Mean gradient (mm. Hg) 49. 8 ± 10. 5 38. 0 ± 15. 5 AR (mod/severe) 11. 7% Peak gradient (mm. Hg) 61. 9 ± 24. 2 Procedur l Characteristics a Valve size implanted Vascular access site 23 mm 25. 8% Transfemoral 25 mm 40. 3% Subclavian 1. 9% 27 mm 33. 9% Transaortic 1. 5% 96. 6%

Baseline & Procedural Characteristics As-Treated Population (N=996) Comorbidities & Baseline Scores Age (Years) Gender (Female) Baseline PPM 80. 8 ± 6. 5 50. 8 13. 3% NYHA Class III or IV 64. 6% STS Score (%) 6. 0 ± 6. 9 Katz Index (frail if <6) 5. 6 ± 1. 0 Echocardiographic Measurements (Core Lab Assessment) AVA (cm 2) 0. 7 ± 0. 2 LVEF (%) MR (mod/severe) 14. 9% Mean gradient (mm. Hg) 49. 8 ± 10. 5 38. 0 ± 15. 5 AR (mod/severe) 11. 7% Peak gradient (mm. Hg) 61. 9 ± 24. 2 Procedur l Characteristics a Valve size implanted Vascular access site 23 mm 25. 8% Transfemoral 25 mm 40. 3% Subclavian 1. 9% 27 mm 33. 9% Transaortic 1. 5% 96. 6%

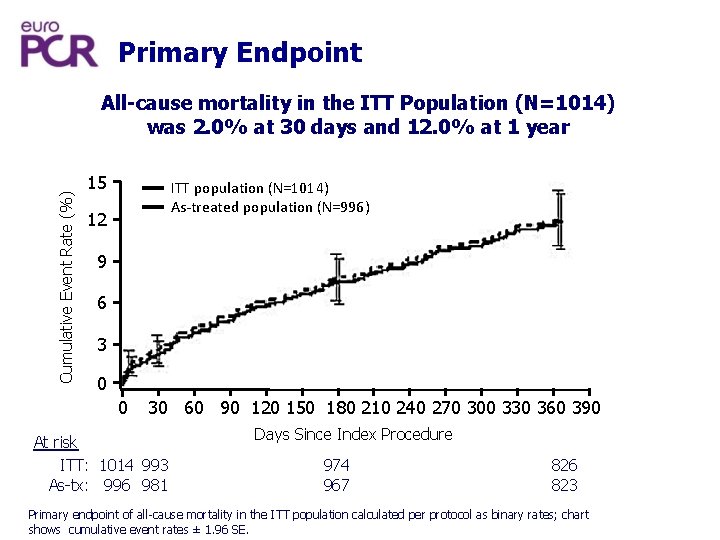
Primary Endpoint Cumulative Event Rate (%) All-cause mortality in the ITT Population (N=1014) was 2. 0% at 30 days and 12. 0% at 1 year 15 ITT population (N=1014) As-treated population (N=996) 12 9 6 3 0 0 30 60 90 120 150 180 210 240 270 300 330 360 390 At risk ITT: 1014 993 As-tx: 996 981 Days Since Index Procedure 974 967 826 823 Primary endpoint of all-cause mortality in the ITT population calculated per protocol as binary rates; chart shows cumulative event rates ± 1. 96 SE.

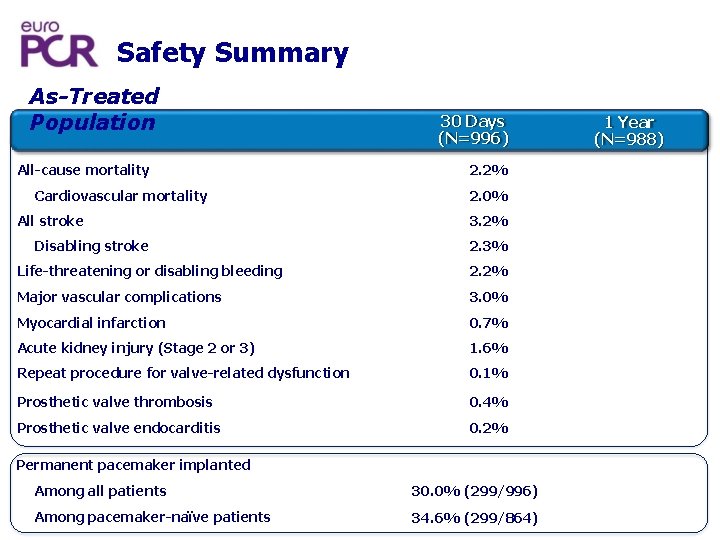
Safety Summary As-Treated Population All-cause mortality Cardiovascular mortality All stroke Disabling stroke 30 Days (N=996) 2. 2% 2. 0% 3. 2% 2. 3% Life-threatening or disabling bleeding 2. 2% Major vascular complications 3. 0% Myocardial infarction 0. 7% Acute kidney injury (Stage 2 or 3) 1. 6% Repeat procedure for valve-related dysfunction 0. 1% Prosthetic valve thrombosis 0. 4% Prosthetic valve endocarditis 0. 2% Permanent pacemaker implanted Among all patients 30. 0% (299/996) Among pacemaker-naïve patients 34. 6% (299/864) 1 Year (N=988)

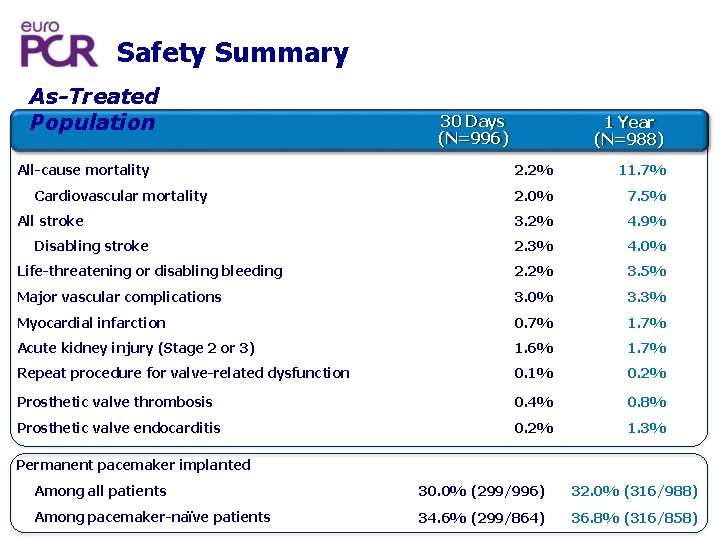
Safety Summary As-Treated Population All-cause mortality 30 Days (N=996) 1 Year (N=988) 2. 2% 11. 7% 2. 0% 7. 5% 3. 2% 4. 9% 2. 3% 4. 0% Life-threatening or disabling bleeding 2. 2% 3. 5% Major vascular complications 3. 0% 3. 3% Myocardial infarction 0. 7% 1. 7% Acute kidney injury (Stage 2 or 3) 1. 6% 1. 7% Repeat procedure for valve-related dysfunction 0. 1% 0. 2% Prosthetic valve thrombosis 0. 4% 0. 8% Prosthetic valve endocarditis 0. 2% 1. 3% Cardiovascular mortality All stroke Disabling stroke Permanent pacemaker implanted Among all patients 30. 0% (299/996) 32. 0% (316/988) Among pacemaker-naïve patients 34. 6% (299/864) 36. 8% (316/858)

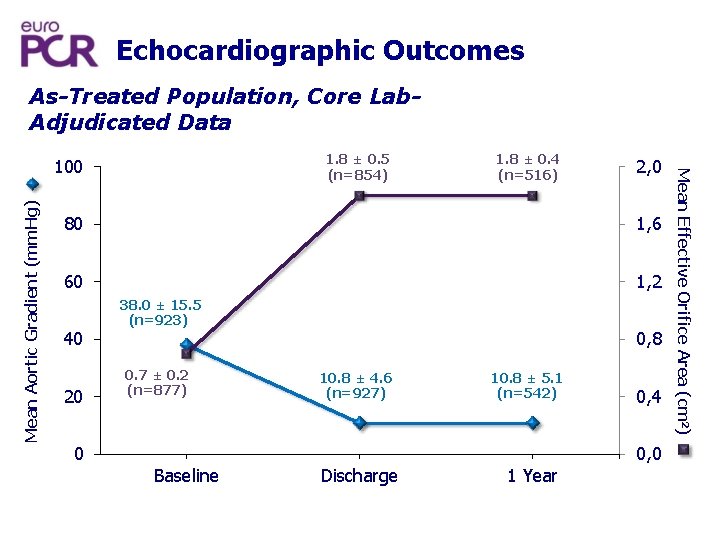
Echocardiographic Outcomes As-Treated Population, Core Lab. Adjudicated Data Mean Aortic Gradient (mm. Hg) 1. 8 ± 0. 4 (n=516) 2, 0 80 1, 6 60 1, 2 40 20 0 38. 0 ± 15. 5 (n=923) 0. 7 ± 0. 2 (n=877) Baseline 0, 8 10. 8 ± 4. 6 (n=927) Discharge 10. 8 ± 5. 1 (n=542) 1 Year 0, 4 0, 0 Mean Effective Orifice Area (cm 2) 1. 8 ± 0. 5 (n=854) 100

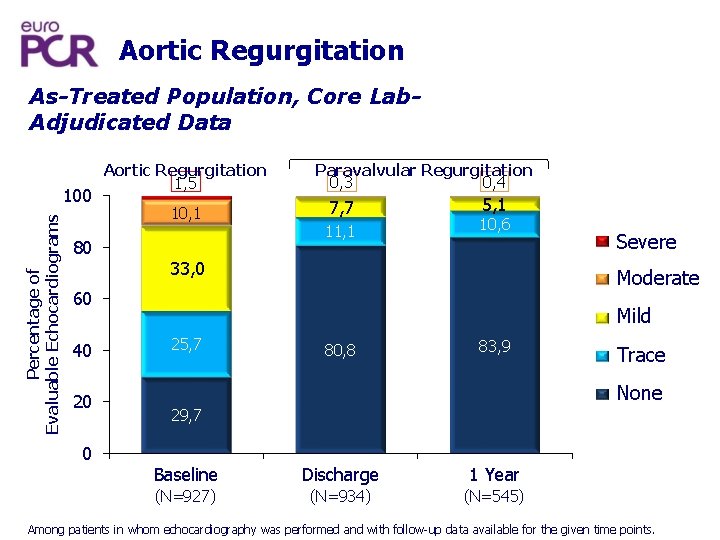
Aortic Regurgitation As-Treated Population, Core Lab. Adjudicated Data Percentage of Evaluable Echocardiograms 100 Aortic Regurgitation 1, 5 10, 1 80 Paravalvular Regurgitation 0, 4 0, 3 7, 7 11, 1 5, 1 10, 6 33, 0 Moderate 60 40 20 0 Severe Mild 25, 7 80, 8 83, 9 Trace None 29, 7 Baseline Discharge 1 Year (N=927) (N=934) (N=545) Among patients in whom echocardiography was performed and with follow-up data available for the given time points.

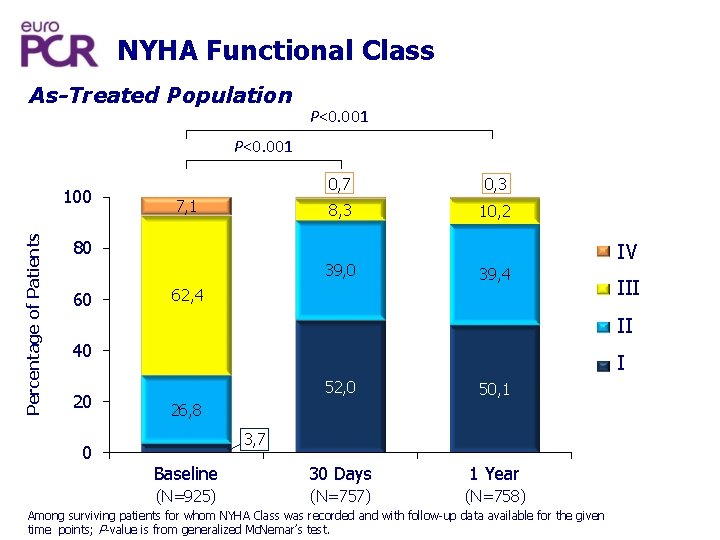
NYHA Functional Class As-Treated Population P<0. 001 Percentage of Patients 100 7, 1 0, 7 0, 3 8, 3 10, 2 80 39, 0 60 IV 39, 4 62, 4 III II 40 20 0 I 52, 0 50, 1 Baseline 30 Days 1 Year (N=925) (N=757) (N=758) 26, 8 3, 7 Among surviving patients for whom NYHA Class was recorded and with follow-up data available for the given time points; P-value is from generalized Mc. Nemar’s test.

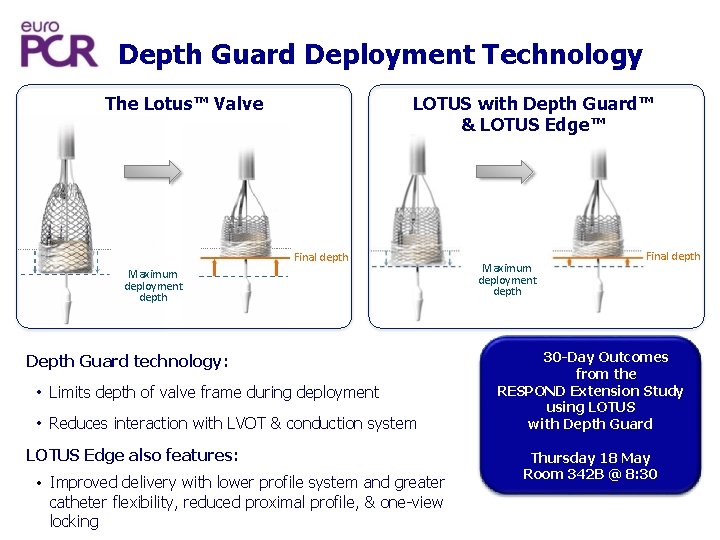
Depth Guard Deployment Technology The Lotus™ Valve LOTUS with Depth Guard™ & LOTUS Edge™ Final depth Maximum deployment depth Depth Guard technology: • Limits depth of valve frame during deployment • Reduces interaction with LVOT & conduction system LOTUS Edge also features: • Improved delivery with lower profile system and greater catheter flexibility, reduced proximal profile, & one-view locking Maximum deployment depth Final depth 30 -Day Outcomes from the RESPOND Extension Study using LOTUS with Depth Guard Thursday 18 May Room 342 B @ 8: 30

Conclusions One-year outcomes from RESPOND confirm the safety and efficacy of the Lotus Valve when used in routine clinical practice • 11. 7% mortality at 1 year post-procedure • Patients maintained excellent valve hemodynamics at 1 year • New permanent pacemaker in 36. 8% of patients • Minimal paravalvular regurgitation (core lab-adjudicated) • 94. 5% of patients with no/trivial PVL at 1 year • No patients with severe PVL; moderate PVL in 0. 4% of patients Pacemaker rates calculated among patients without a prior pacemaker at baseline.