ONEYEAR OUTCOMES OLAF WENDLER MD PHD FRCS ON










- Slides: 22

ONE-YEAR OUTCOMES OLAF WENDLER, MD PHD FRCS ON BEHALF OF THE SOURCE 3 INVESTIGATORS

Potential Conflicts of Interest Speaker's name: Olaf Wendler I have the following potential conflicts of interest to report: Consultant to Edwards Lifesciences

Design and Execution Patients Enrollment Centres Countries Access Routes THV Clinical Endpoint Adjudication Follow-up 1946 July 2014 – Oct 2015 80 10 TF, TAo, SC, TC SAPIEN 3™ 23 mm, 26 mm, 29 mm Independent CEC, VARC 2 Definitions 30 Days, Annually to 5 Years

Study Administration Principal Steering Committee Investigators Olaf Wendler, MD Helmut Baumgartner, MD King’s College Hospital, London, UK Alec Vahanian, MD University Clinics, Muenster, Germany Nicolas Dumonteil, MD Clinique Pasteur, Toulouse, France Hôpital Bichat, Paris, France Franz-Josef Neumann, MD Clinical Events Committee Chairman: Philip Urban, MD Gerhard Schymik, MD Hôpital de La Tour, Meyrin, Switzerland University Heart Center, Bad Krozingen, Germany Municipal Hospital, Karlsruhe, Germany Giuseppe Tarantini, MD Policlinico Universitario, Padova, Italy Echo Sub-study Core Laboratory José Luis Zamorano, MD University Hospital Ramon y Cajal, Madrid, Spain Hendrik Treede, MD University Clinics, Halle, Germany

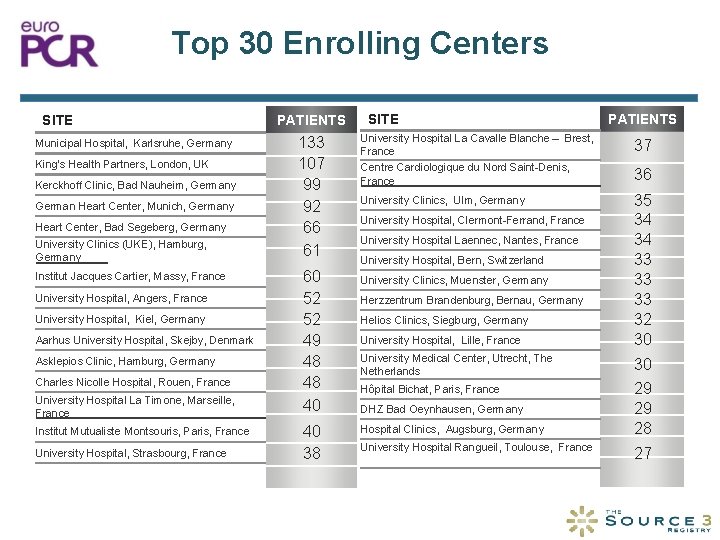
Top 30 Enrolling Centers SITE Municipal Hospital, Karlsruhe, Germany King’s Health Partners, London, UK Kerckhoff Clinic, Bad Nauheim, Germany German Heart Center, Munich, Germany Heart Center, Bad Segeberg, Germany University Clinics (UKE), Hamburg, Germany Institut Jacques Cartier, Massy, France University Hospital, Angers, France University Hospital, Kiel, Germany Aarhus University Hospital, Skejby, Denmark Asklepios Clinic, Hamburg, Germany Charles Nicolle Hospital, Rouen, France University Hospital La Timone, Marseille, France Institut Mutualiste Montsouris, Paris, France University Hospital, Strasbourg, France PATIENTS 133 107 99 92 66 61 60 52 52 49 48 48 40 40 38 SITE PATIENTS University Hospital La Cavalle Blanche – Brest, France Centre Cardiologique du Nord Saint-Denis, France 37 University Clinics, Ulm, Germany 35 34 34 33 33 33 32 30 University Hospital, Clermont-Ferrand, France University Hospital Laennec, Nantes, France University Hospital, Bern, Switzerland University Clinics, Muenster, Germany Herzzentrum Brandenburg, Bernau, Germany Helios Clinics, Siegburg, Germany University Hospital, Lille, France University Medical Center, Utrecht, The Netherlands Hôpital Bichat, Paris, France DHZ Bad Oeynhausen, Germany Hospital Clinics, Augsburg, Germany University Hospital Rangueil, Toulouse, France 36 30 29 29 28 27

Baseline Characteristics Age (years ±SD) Female (%) BMI (mean ±SD) Total TF Non-TF N=1946 N=1694 N=251 81. 6 ± 6. 7 81. 7 ± 6. 7 80. 8 ± 6. 4 0. 025 48 49. 2 39. 7 0. 006 27. 0 ± 5. 0 27. 0 ± 5. 1 26. 82 ± 4. 6 p-value 0. 75 Chronic Lung Disease (%) 11. 9 11 17. 9 0. 003 Renal Insufficiency (%) Log Euro. SCORE (med) 27. 5 26. 9 31. 7 0. 11 14. 42 13. 96 17. 83 <0. 001 <10 (%) 29. 0 30. 6 18. 5 <0. 001 >30 (%) 15. 8 14. 5 24. 6 <0. 001 (Wendler et al. Circulation 2017)

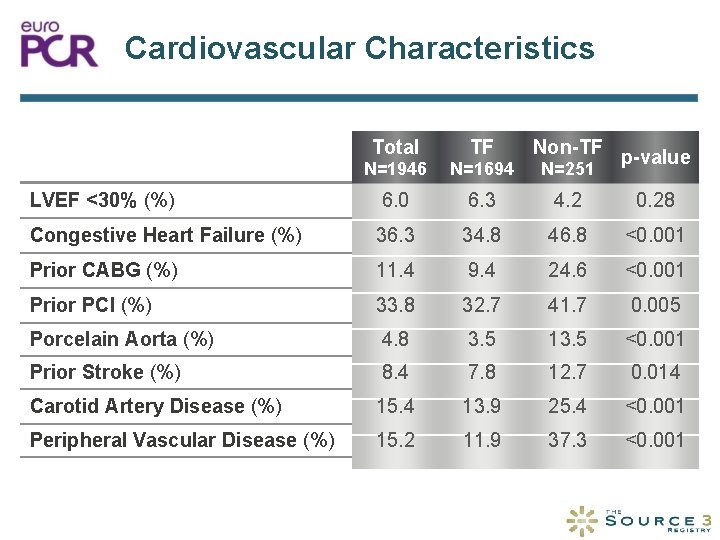
Cardiovascular Characteristics Total TF Non-TF N=1946 N=1694 N=251 LVEF <30% (%) 6. 0 6. 3 4. 2 0. 28 Congestive Heart Failure (%) 36. 3 34. 8 46. 8 <0. 001 Prior CABG (%) 11. 4 9. 4 24. 6 <0. 001 Prior PCI (%) 33. 8 32. 7 41. 7 0. 005 Porcelain Aorta (%) 4. 8 3. 5 13. 5 <0. 001 Prior Stroke (%) 8. 4 7. 8 12. 7 0. 014 Carotid Artery Disease (%) 15. 4 13. 9 25. 4 <0. 001 Peripheral Vascular Disease (%) 15. 2 11. 9 37. 3 <0. 001 p-value

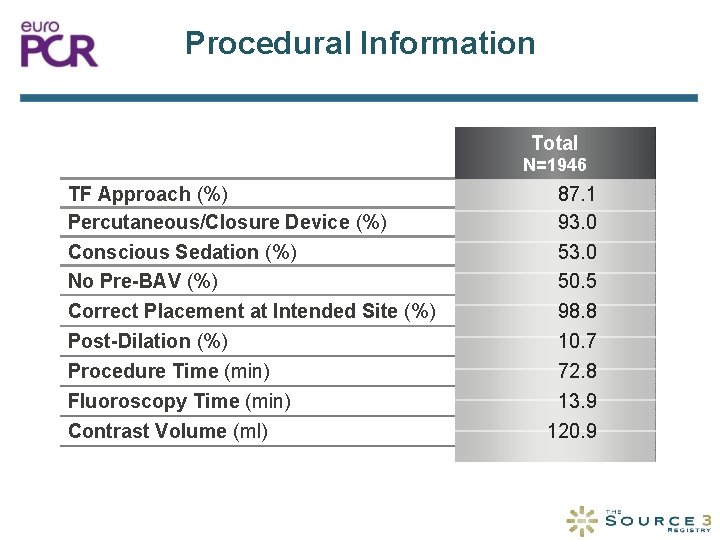
Procedural Information Total N=1946 TF Approach (%) Percutaneous/Closure Device (%) Conscious Sedation (%) No Pre-BAV (%) 87. 1 93. 0 50. 5 Correct Placement at Intended Site (%) 98. 8 Post-Dilation (%) Procedure Time (min) Fluoroscopy Time (min) 10. 7 72. 8 13. 9 Contrast Volume (ml) 120. 9

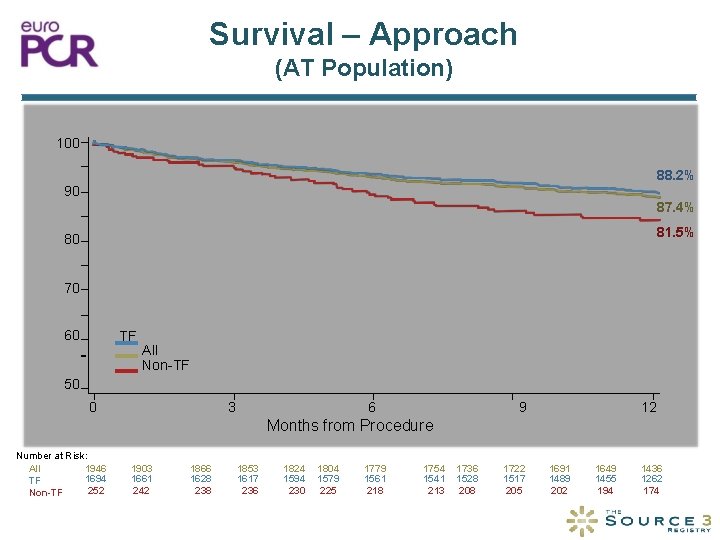
Survival – Approach (AT Population) 100 88. 2% 90 Survival (%) 87. 4% 81. 5% 80 70 60 TF All Non-TF 50 0 3 6 9 12 Months from Procedure Number at Risk: 1946 All 1694 TF 252 Non-TF 1903 1661 242 1866 1628 238 1853 1617 236 1824 1594 230 1804 1579 225 1779 1561 218 1754 1541 213 1736 1528 208 1722 1517 205 1691 1489 202 1649 1455 194 1436 1262 174

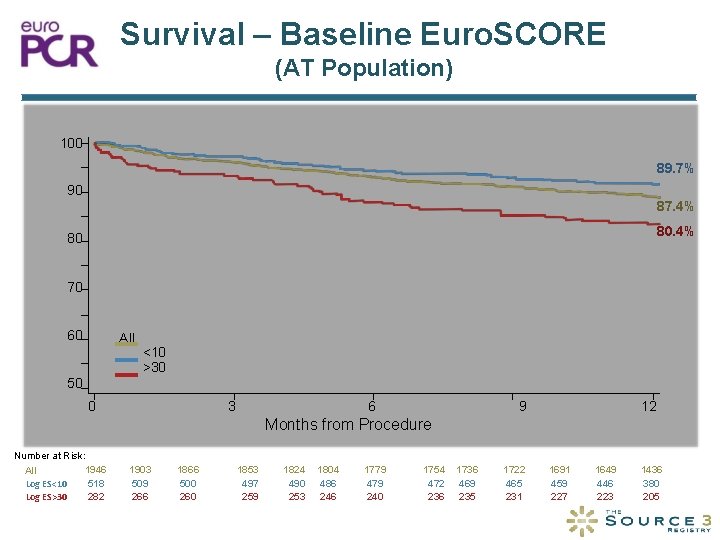
Survival – Baseline Euro. SCORE (AT Population) 100 89. 7% 90 Survival (%) 87. 4% 80 70 60 All <10 >30 50 0 3 6 9 12 Months from Procedure Number at Risk: 1946 All Log ES<10 518 Log ES>30 282 1903 509 266 1866 500 260 1853 497 259 1824 490 253 1804 486 246 1779 479 240 1754 472 236 1736 469 235 1722 465 231 1691 459 227 1649 446 223 1436 380 205

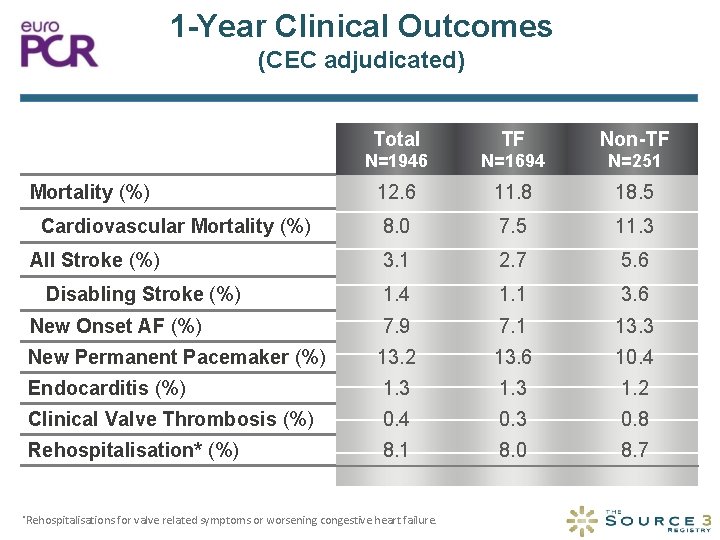
1 -Year Clinical Outcomes (CEC adjudicated) Total TF Non-TF N=1946 N=1694 N=251 12. 6 11. 8 18. 5 8. 0 7. 5 11. 3 3. 1 2. 7 5. 6 1. 4 1. 1 3. 6 New Onset AF (%) 7. 9 7. 1 13. 3 New Permanent Pacemaker (%) 13. 2 13. 6 10. 4 Endocarditis (%) 1. 3 1. 2 Clinical Valve Thrombosis (%) 0. 4 0. 3 0. 8 Rehospitalisation* (%) 8. 1 8. 0 8. 7 Mortality (%) Cardiovascular Mortality (%) All Stroke (%) Disabling Stroke (%) *Rehospitalisations for valve related symptoms or worsening congestive heart failure.

NYHA Classification Total§ Non-TF§ % of Population 100 Class IV 90 Class III 80 Class II Class I 70 60 50 40 30 20 10 0 Baseline §p-value 30 -Days One-Year Baseline <0. 001: Baseline vs. 30 -Days and 1 -Year 30 -Days One-Year

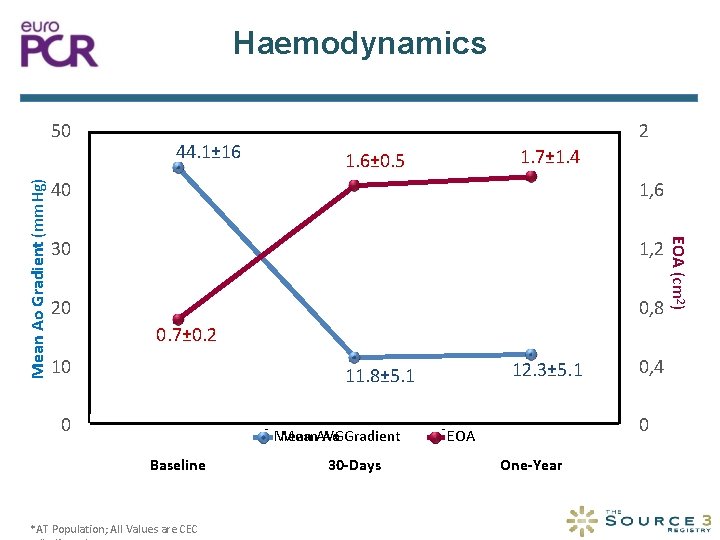
Haemodynamics 44. 1± 16 2 1. 7± 1. 4 1. 6± 0. 5 40 1, 6 30 1, 2 20 0, 8 0. 7± 0. 2 10 12. 3± 5. 1 11. 8± 5. 1 0 Mean. AVG Ao Gradient Mean Baseline *AT Population; All Values are CEC 30 -Days 0, 4 0 EOA One-Year EOA (cm 2) Mean Ao Gradient (mm. Hg) 50

Paravalvular Regurgitation Total§ TF§ Non-TF¶ Severe Paired Analyses p-values between discharge and one-year are § p-value<0. 001 and ¶p-value=0. 19

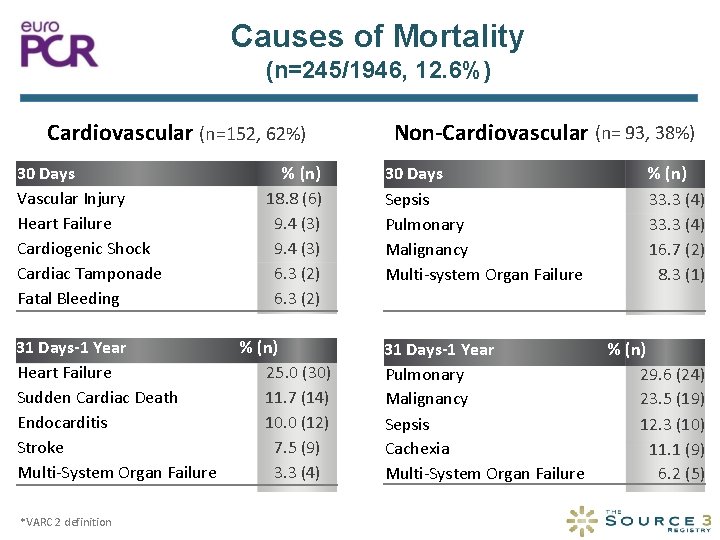
Causes of Mortality (n=245/1946, 12. 6%) Cardiovascular (n=152, 62%) 30 Days Vascular Injury Heart Failure Cardiogenic Shock Cardiac Tamponade Fatal Bleeding 31 Days-1 Year Heart Failure Sudden Cardiac Death Endocarditis Stroke Multi-System Organ Failure *VARC 2 definition % (n) 18. 8 (6) 9. 4 (3) 6. 3 (2) % (n) 25. 0 (30) 11. 7 (14) 10. 0 (12) 7. 5 (9) 3. 3 (4) Non-Cardiovascular (n= 93, 38%) 30 Days Sepsis Pulmonary Malignancy Multi-system Organ Failure % (n) 33. 3 (4) 16. 7 (2) 8. 3 (1) 31 Days-1 Year Pulmonary Malignancy Sepsis Cachexia Multi-System Organ Failure % (n) 29. 6 (24) 23. 5 (19) 12. 3 (10) 11. 1 (9) 6. 2 (5)

Peri-Procedural Baseline Univariate Predictors 1 -Year Mortality Variable HR* p-value Tricuspid Regurgitation Mod-Severe NYHA IV Renal Insufficiency Atrial Fibrillation Log Euro. SCORE Mean Aortic Valve Gradient Body Mass Index Procedure Time (Skin-Skin) Transfemoral Access Acute Kidney Injury (≤ 7 d) Major Vascular Complications (≤ 30 d) ICU Length of Stay Days from Implant to Discharge 2. 19 2. 37 2. 04 1. 77 1. 02 0. 98 0. 97 1. 00 0. 61 3. 13 2. 59 1. 07 1. 04 <0. 001 *HR: Hazard Ratio; All variables with p-value < 0. 2 were included in the multivariable analyses 0. 109 <0. 001 0. 021 0. 150 0. 003 <0. 001

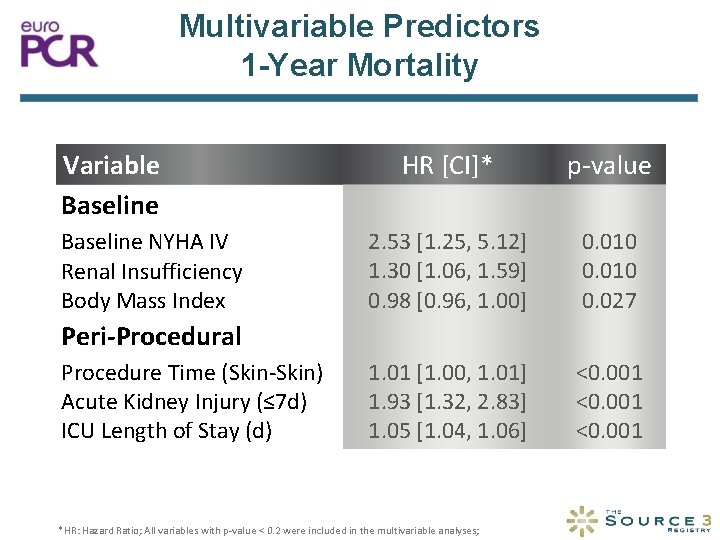
Multivariable Predictors 1 -Year Mortality Variable Baseline NYHA IV Renal Insufficiency Body Mass Index HR [CI]* p-value 2. 53 [1. 25, 5. 12] 1. 30 [1. 06, 1. 59] 0. 98 [0. 96, 1. 00] 0. 010 0. 027 1. 01 [1. 00, 1. 01] 1. 93 [1. 32, 2. 83] 1. 05 [1. 04, 1. 06] <0. 001 Peri-Procedural Procedure Time (Skin-Skin) Acute Kidney Injury (≤ 7 d) ICU Length of Stay (d) *HR: Hazard Ratio; All variables with p-value < 0. 2 were included in the multivariable analyses;

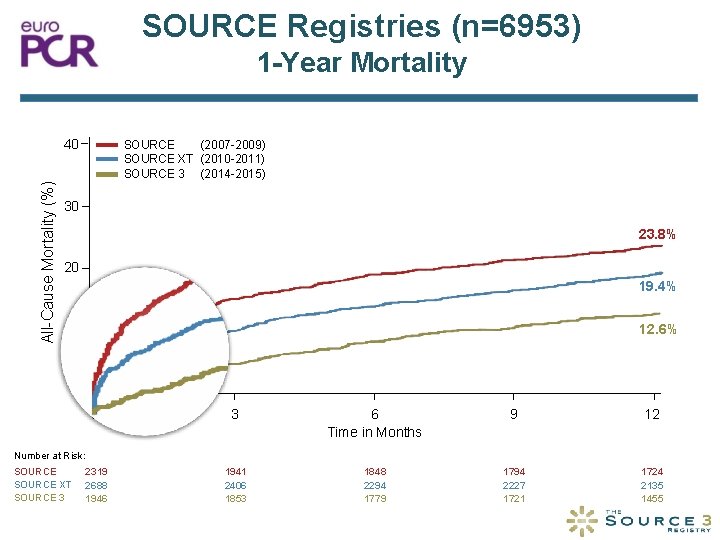
SOURCE Registries (n=6953) 1 -Year Mortality All-Cause Mortality (%) 40 SOURCE (2007 -2009) SOURCE XT (2010 -2011) SOURCE 3 (2014 -2015) 30 23. 8% 20 19. 4% 12. 6% 10 0 0 3 6 Time in Months 9 12 2319 2688 1946 1941 2406 1853 1848 2294 1779 1794 2227 1721 1724 2135 1455 Number at Risk: SOURCE XT SOURCE 3

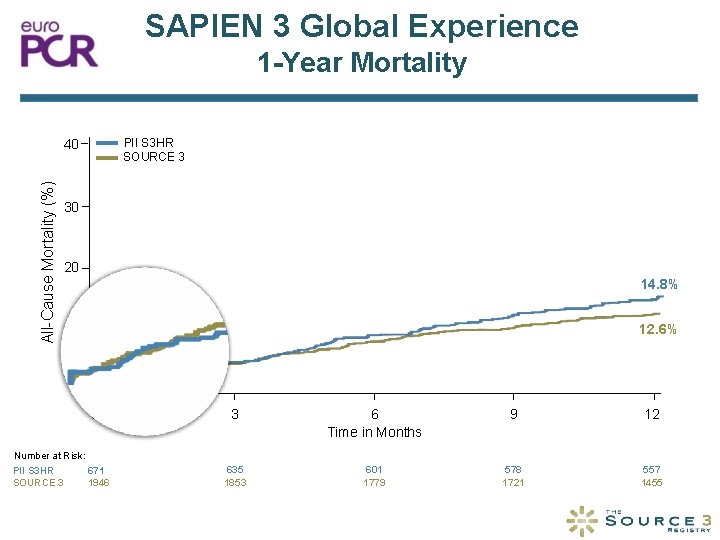
SAPIEN 3 Global Experience 1 -Year Mortality PII S 3 HR SOURCE 3 All-Cause Mortality (%) 40 30 20 14. 8% 12. 6% 10 0 0 Number at Risk: 671 PII S 3 HR SOURCE 3 1946 3 6 Time in Months 9 12 635 1853 601 1779 578 1721 557 1455

Conclusions • The low peri-procedural complication rate at 30 days • • reported in SOURCE 3 has resulted in excellent survival at 1 -year. After 1 -year more than 90% of patients are in NYHA I/II. No severe PVL has been observed and the rate of moderate PVL is low (2. 6%). NYHA IV and renal insufficiency are the strongest baseline characteristics to predict 1 -year mortality in the multivariable analysis. These European results from the largest reported registry with SAPIEN 3 are consistent with 1 -year outcomes observed in North American trials.

Implications • The clinical outcomes of SOURCE 3 highlight the • • improvements of TAVI treatment in general and the SAPIEN 3 THV in particular. The similarities of 30 -day and 1 -year outcomes of the global experience may indicate that the present balloon - expandable technology has become less dependent of operator variability. SOURCE 3 provides further evidence on TAVI, however, longer follow-up is vital before TAVI is rolled out to low risk patients.

Publication Accepted for Fast Track Publication
 Professor olaf wendler
Professor olaf wendler Titul frcs(t)
Titul frcs(t) Frcs general surgery questions
Frcs general surgery questions Frcsed meaning
Frcsed meaning Franz wendler
Franz wendler Tiffany wendler
Tiffany wendler Maria emilia wendler muller
Maria emilia wendler muller Bbwe
Bbwe Olaf schaaf
Olaf schaaf Student information system st olaf
Student information system st olaf Olaf diegel
Olaf diegel Erwin olaf biografie
Erwin olaf biografie 247remote
247remote Olaf lobermeier
Olaf lobermeier St olaf nmr
St olaf nmr Rubric maken
Rubric maken Olaf booy
Olaf booy Olaf schneider schornsteinfeger
Olaf schneider schornsteinfeger Olaf blanke out of body
Olaf blanke out of body Ivko magister
Ivko magister Olaf d
Olaf d Olaf heinicke
Olaf heinicke Olaf medenbach
Olaf medenbach