The Quantum Mechanical Model of Light Max Planck





- Slides: 14

The Quantum Mechanical Model of Light Max Planck was the first to propose that the energy an electron can absorb, and thus emit, is limited to specific quantities of energy called quanta.

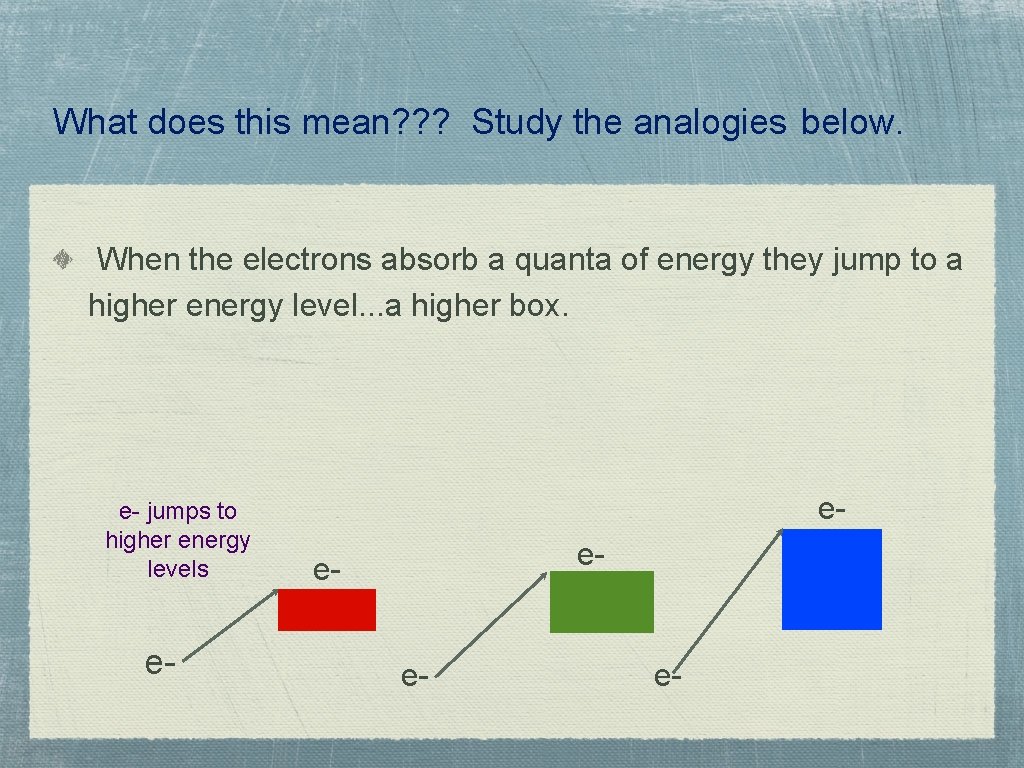
What does this mean? ? ? Study the analogies below. When the electrons absorb a quanta of energy they jump to a higher energy level. . . a higher box. e- jumps to higher energy levels e- ee- e-

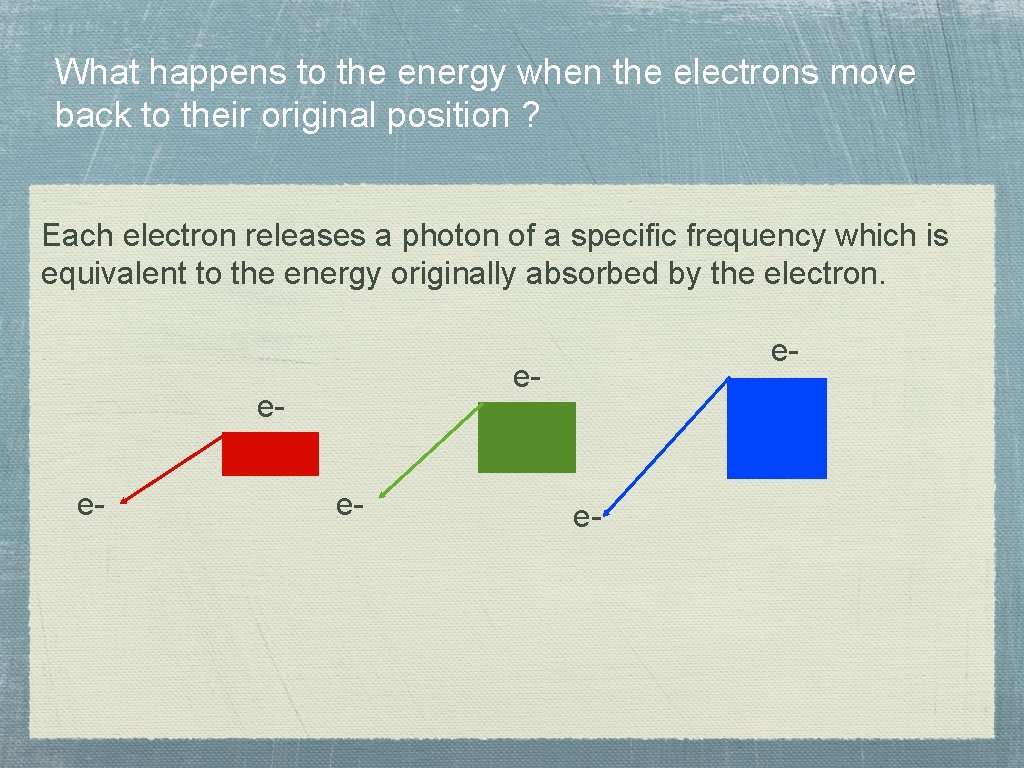
What happens to the energy when the electrons move back to their original position ? Each electron releases a photon of a specific frequency which is equivalent to the energy originally absorbed by the electron. e- e-

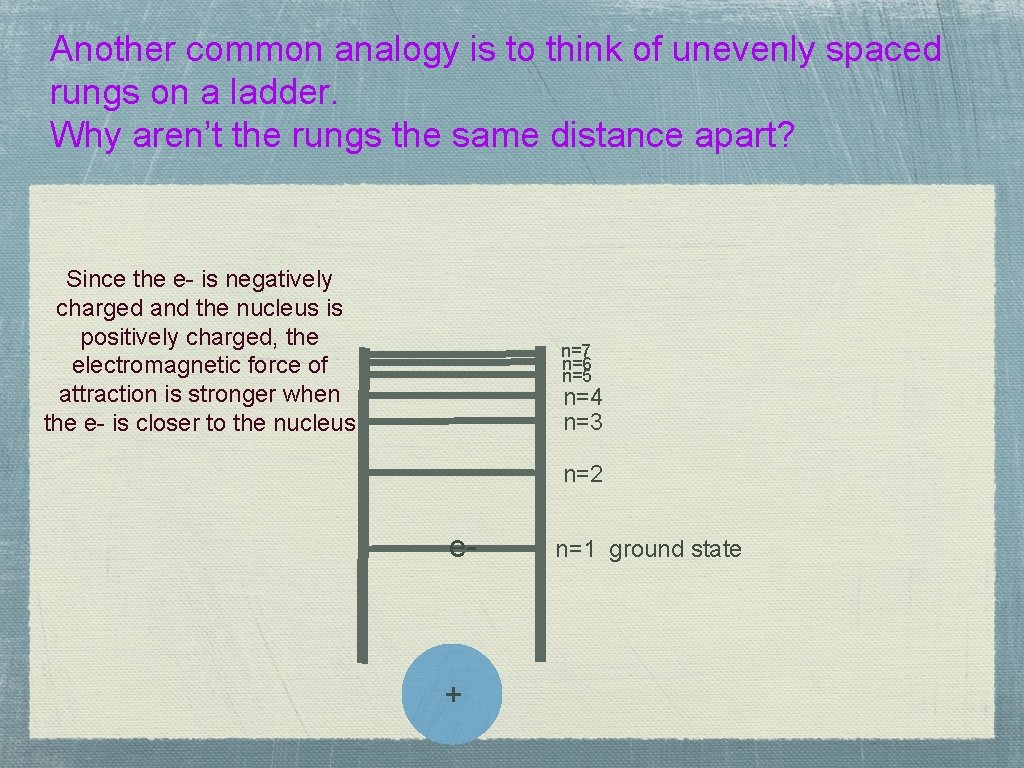
Another common analogy is to think of unevenly spaced rungs on a ladder. Why aren’t the rungs the same distance apart? Since the e- is negatively charged and the nucleus is positively charged, the electromagnetic force of attraction is stronger when the e- is closer to the nucleus n=7 n=6 n=5 n=4 n=3 n=2 e- + n=1 ground state

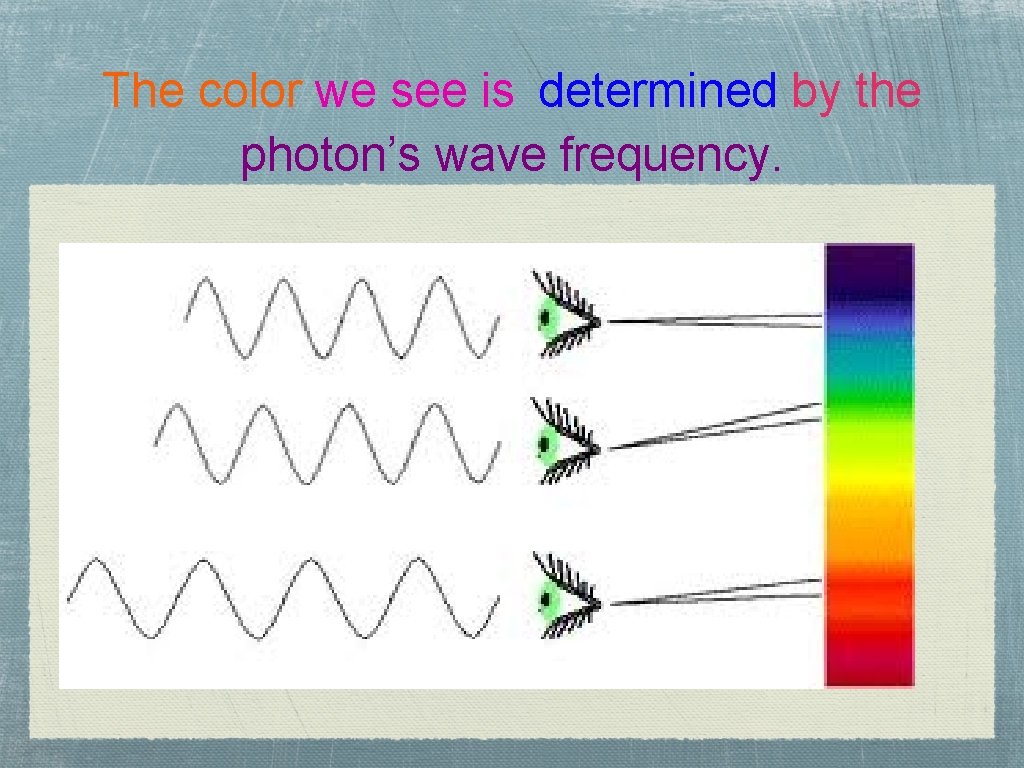
The color we see is determined by the photon’s wave frequency.

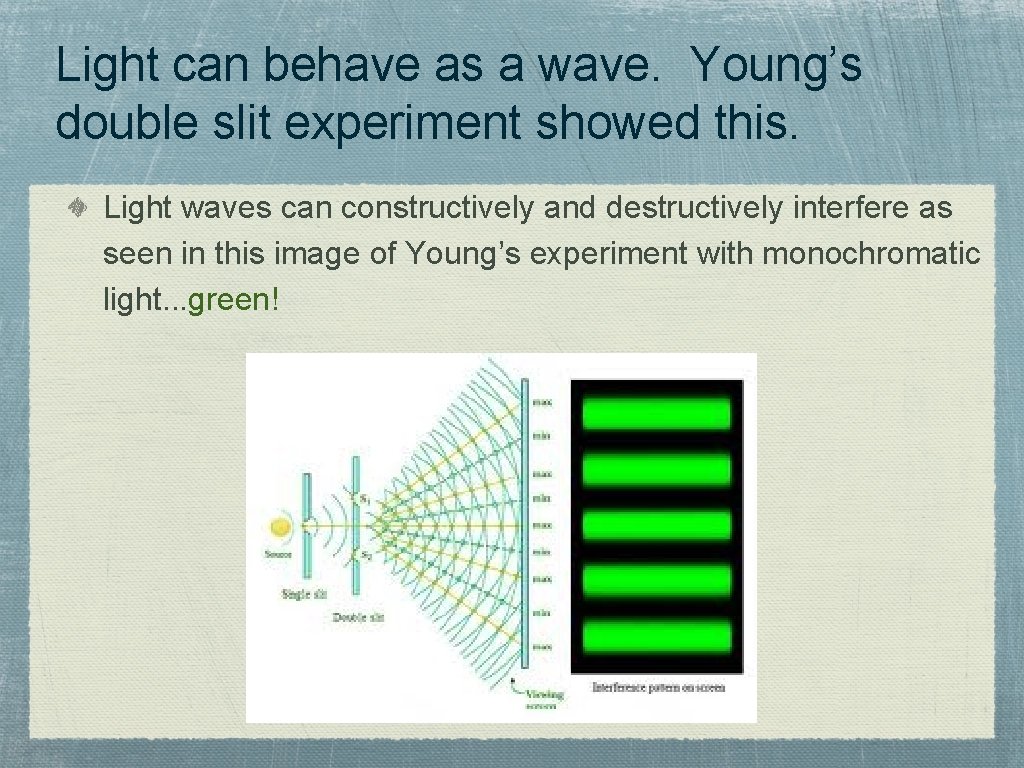
Light can behave as a wave. Young’s double slit experiment showed this. Light waves can constructively and destructively interfere as seen in this image of Young’s experiment with monochromatic light. . . green!

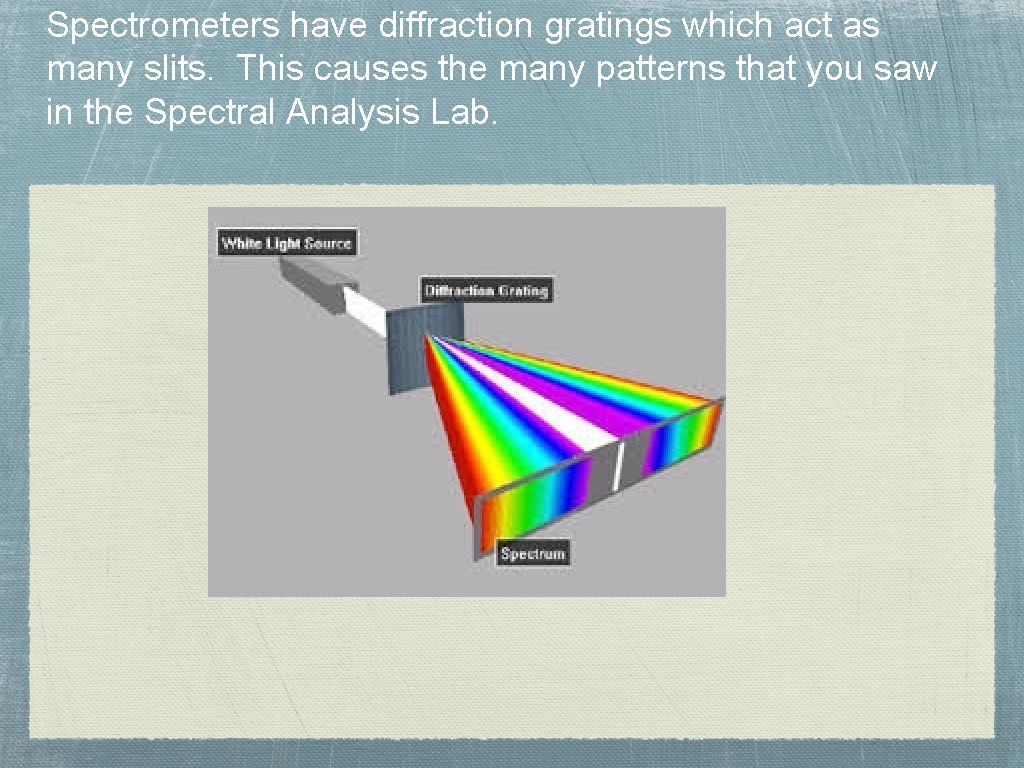
Spectrometers have diffraction gratings which act as many slits. This causes the many patterns that you saw in the Spectral Analysis Lab.

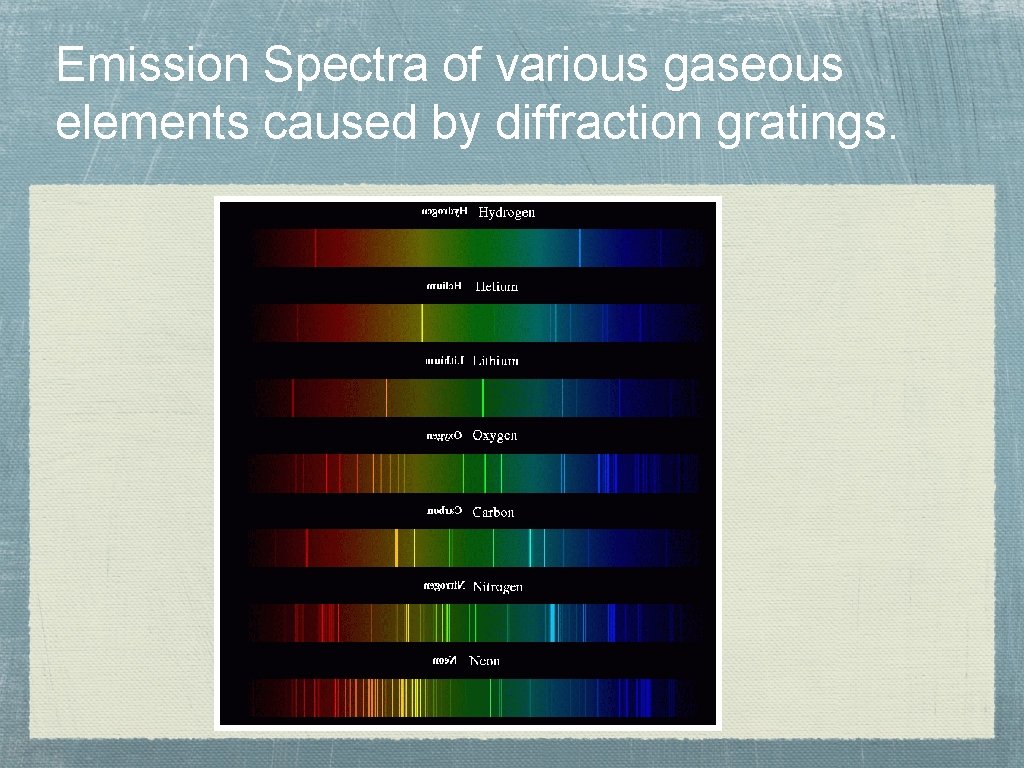
Emission Spectra of various gaseous elements caused by diffraction gratings.

Einstein won the Nobel Prize for Physics in 1921 for his Photoelectric Effect Experiment Text

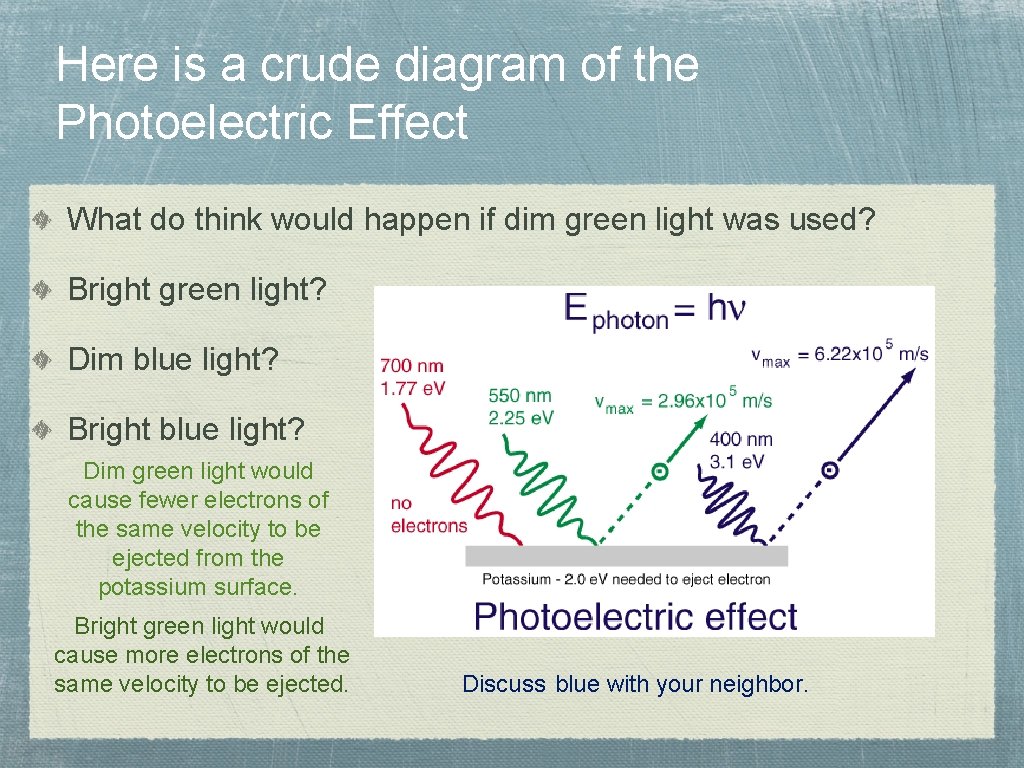
Here is a crude diagram of the Photoelectric Effect What do think would happen if dim green light was used? Bright green light? Dim blue light? Bright blue light? Dim green light would cause fewer electrons of the same velocity to be ejected from the potassium surface. Bright green light would cause more electrons of the same velocity to be ejected. Discuss blue with your neighbor.

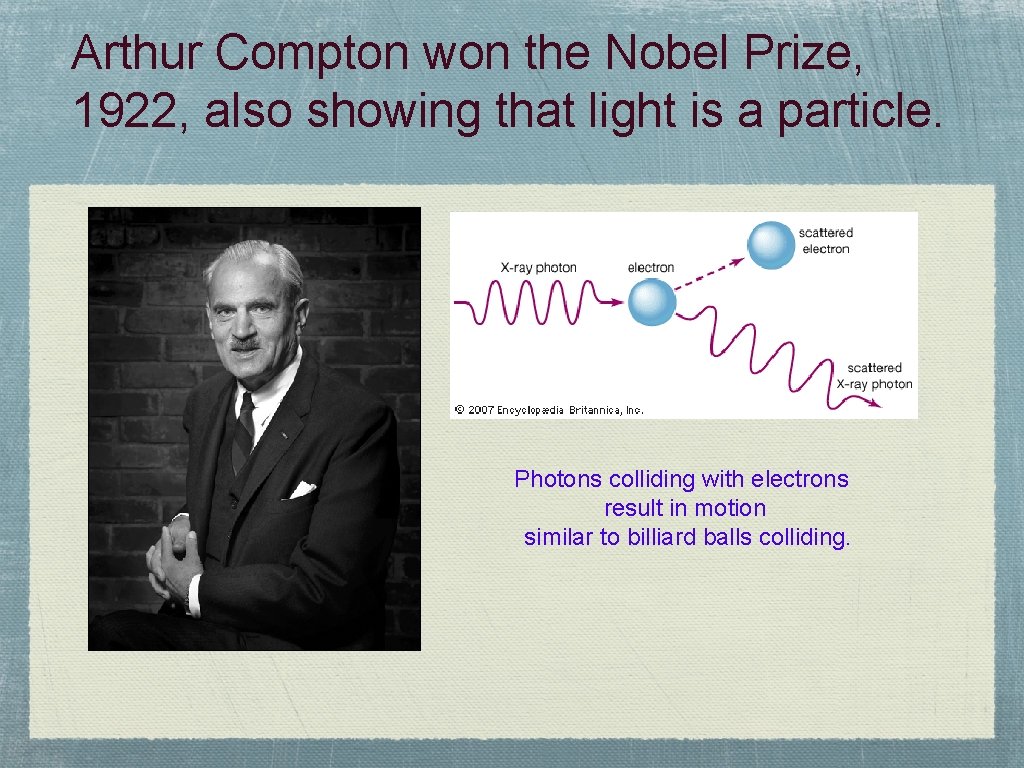
Arthur Compton won the Nobel Prize, 1922, also showing that light is a particle. Photons colliding with electrons result in motion similar to billiard balls colliding.

Victor De. Broglie won the Nobel Prize in 1924. He showed that all matter, including you, is a wave as well as a particle! As you can see by the equation, your frequency would be super large, so your wavelength is super-duper small.

Werner Heisenberg won the Nobel Prize in 1932 for his Uncertainty Principle. The Uncertainty Principle states that you can’t know an electron’s location & its speed at the same time. Here’s a joke! Heisenberg was driving very fast on the Autobahn when a policeman pulled him over. The policeman asked him, “Do you know how fast you were going? ” Heisenberg responded, “No, but I know where I am!” Text

This presentation was meant for review and to stimulate your brain about light. Isn’t it fascinating? ?