The Particle Model Models in Science Have you









- Slides: 22

The Particle Model


Models in Science Have you ever put together a model car or airplane? If so, in what ways are the model and the real object the same? In what ways are they different? Scientists often use models to help them describe an idea that is difficult to see or understand without using a model. In science, a model is a representation of an object, idea, or event and helps everyone in the understanding of a more complex concept.

What is the Particle Model? • The Particle Model (PM) helps us to explain how matter is structured. Because we can not actually see the microscopic world, we must use a model to visualize what matter looks like and how it behaves. • The PM states that all matter is made up of tiny particles that are always in motion. These particles are so small that no one can see them without the help from technology.

Key Ideas about the Particle Model • All matter is made up of tiny particles (most visual models use tiny balls or marbles to represent the particles) • The particles that make up a single substance are all the same even if the substance is in different states of matter (solid, liquid, or gas). Only the connections between the particles changes when the state of matter changes. • Particles are always moving (the only exception would be at absolute zero)

Solids in the Particle Model • Solids are made of particles that vibrate around a fixed spot. They will vibrate in all directions, but are held in place by connections (sometimes called bonds) between the particles. • These connections keep solids closely packed together and limits their ability to move around.

Liquids in the Particle Model • Liquids are made of particles just like solids, but the connections between the particles in a liquid are much weaker. • Because these connections are weaker, the particles in a liquid can move past one another giving liquids the ability to flow.

Gases in the Particle Model • Gases are made up of particles as well. The particles that make up a gas move about the container as much as they want since there are no connections between the particles. • The particles in a gas usually move away from one another to fill the entire container. The “container” may be a closed bottle or the planet Earth, which is closed by the atmosphere.

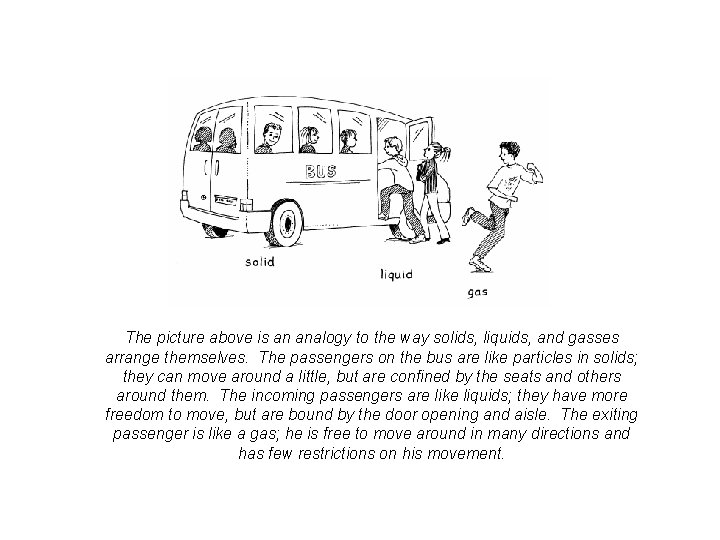
The picture above is an analogy to the way solids, liquids, and gasses arrange themselves. The passengers on the bus are like particles in solids; they can move around a little, but are confined by the seats and others around them. The incoming passengers are like liquids; they have more freedom to move, but are bound by the door opening and aisle. The exiting passenger is like a gas; he is free to move around in many directions and has few restrictions on his movement.

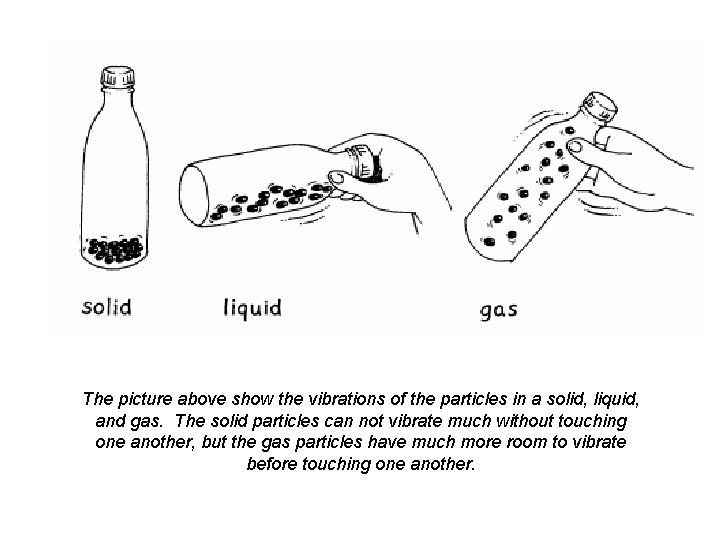
The picture above show the vibrations of the particles in a solid, liquid, and gas. The solid particles can not vibrate much without touching one another, but the gas particles have much more room to vibrate before touching one another.

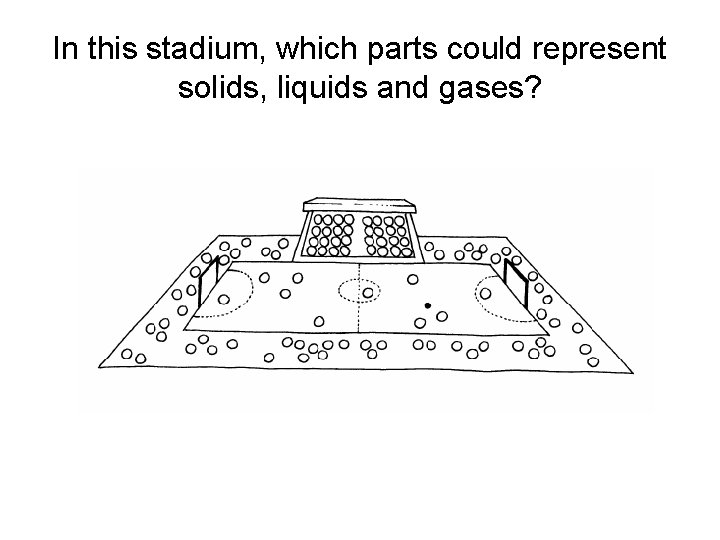
In this stadium, which parts could represent solids, liquids and gases?

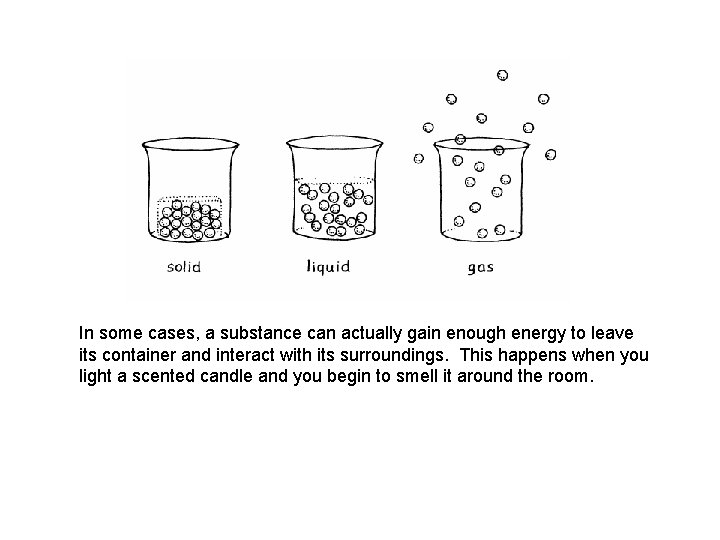
In some cases, a substance can actually gain enough energy to leave its container and interact with its surroundings. This happens when you light a scented candle and you begin to smell it around the room.

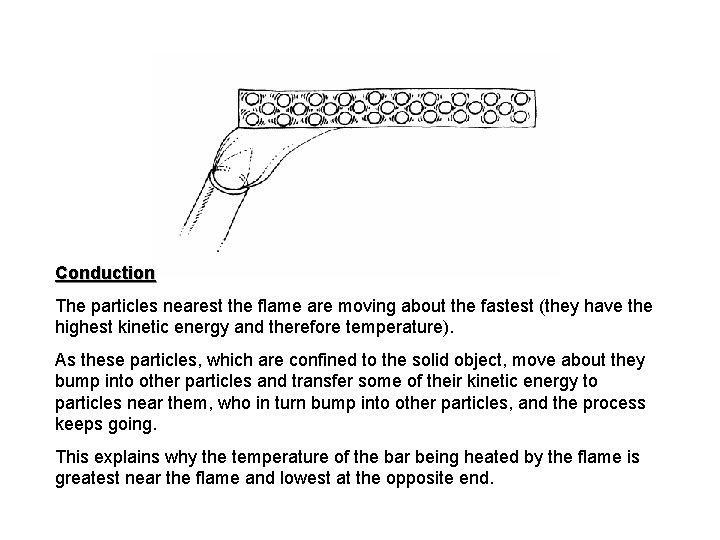
Conduction in the Particle Model • Conduction is the transfer of heat energy by the interactions of particles. • Particles, usually in a solid, nearest the heat source move around more and more. Consequently, they bump into the other particles nearest them and transfer some of their motion energy to them, who them bump into more and more of the particles that make up the substance. • Good conductors are materials where the particles are close together and can interact easily.

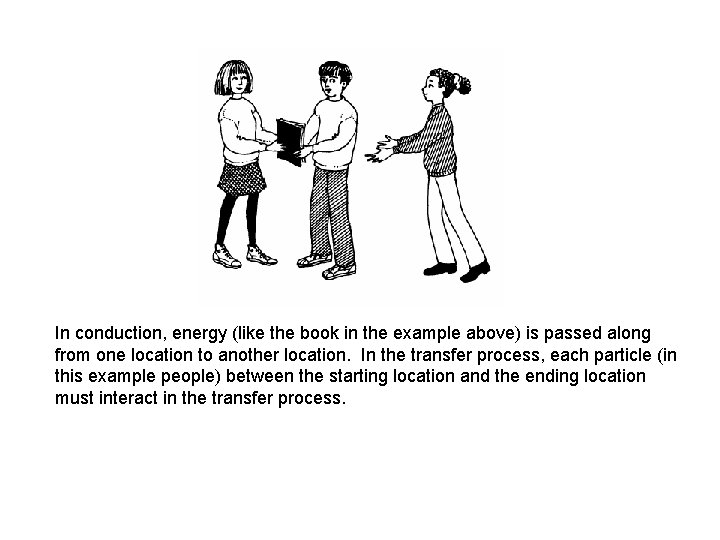
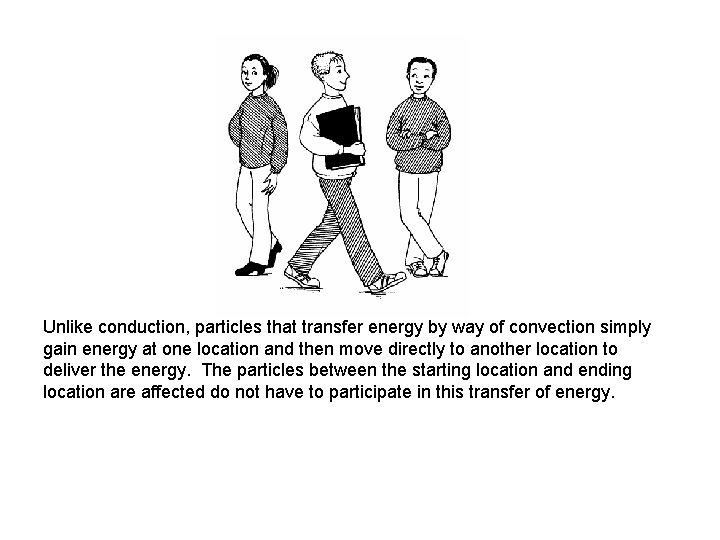
In conduction, energy (like the book in the example above) is passed along from one location to another location. In the transfer process, each particle (in this example people) between the starting location and the ending location must interact in the transfer process.

Conduction The particles nearest the flame are moving about the fastest (they have the highest kinetic energy and therefore temperature). As these particles, which are confined to the solid object, move about they bump into other particles and transfer some of their kinetic energy to particles near them, who in turn bump into other particles, and the process keeps going. This explains why the temperature of the bar being heated by the flame is greatest near the flame and lowest at the opposite end.

Convection in the Particle Model • Convection is the transfer of heat energy by the movement of particles and their interactions. • Only gases can transfer energy by way of convection because the particles are not connected to one another.

Unlike conduction, particles that transfer energy by way of convection simply gain energy at one location and then move directly to another location to deliver the energy. The particles between the starting location and ending location are affected do not have to participate in this transfer of energy.

Changes of State in the Particle Model • As heat energy is added to a substance, the particles’ movement increases. The particles spread out because of this increase in energy • It is the space between particles that gets larger and NOT the particles themselves. • It takes energy to make the particles vibrate more AND it takes energy to weaken or destroy the connections between particles. This is the reason that adding heat energy does not always increase the temperature of the substance.

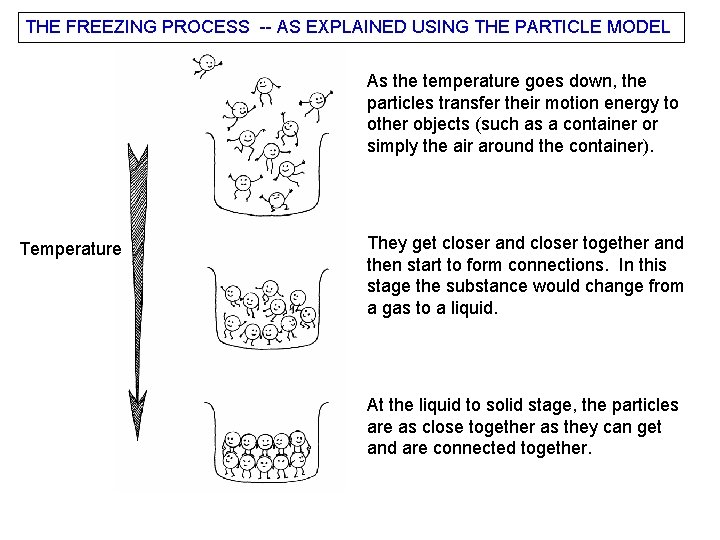
THE FREEZING PROCESS -- AS EXPLAINED USING THE PARTICLE MODEL As the temperature goes down, the particles transfer their motion energy to other objects (such as a container or simply the air around the container). Temperature They get closer and closer together and then start to form connections. In this stage the substance would change from a gas to a liquid. At the liquid to solid stage, the particles are as close together as they can get and are connected together.

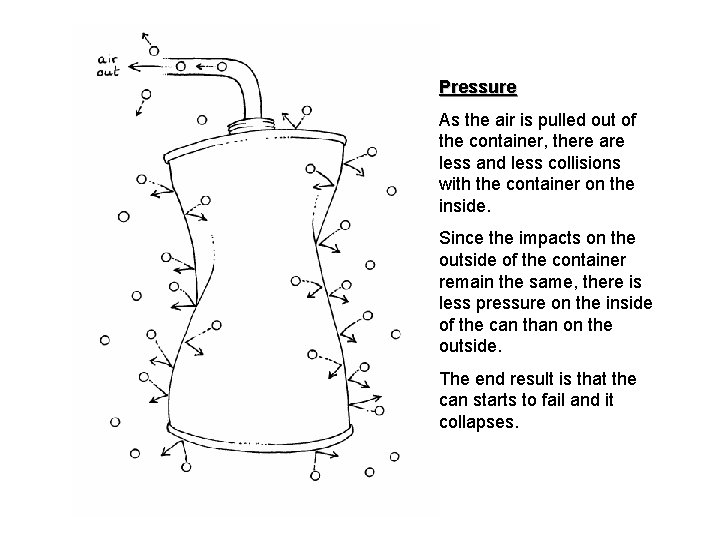
Pressure in the Particle Model • Gas particles are moving around all the time and spread out to fill their container. • As the particles bump into their container, the collisions result in a force being put on the particle to change its direction and an equal force being put on the container. • The pressure in (or on) a container is affected by the number of particles in and out of the container, the number of impacts, and the strength of the impacts.

Pressure As the air is pulled out of the container, there are less and less collisions with the container on the inside. Since the impacts on the outside of the container remain the same, there is less pressure on the inside of the can than on the outside. The end result is that the can starts to fail and it collapses.

© The Delaware Science Coalition/DE Department of Education/Moyer © On the Ball, by Smith, Tregido, Wells, Evans, Radcliffe, and Eaton; University of Southampton