The microwave spectrum of partially deuterated species of





- Slides: 19

The microwave spectrum of partially deuterated species of dimethyl ether a b D. Lauvergnat, L. Margulès, R. A. Motyenko, c d J. -C. Guillemin, and L. H. Coudert b a LCP, CNRS/Université Paris-Sud, Orsay, France b Ph. LAM, CNRS/Université des Sciences et Technologies de Lille 1 Villeneuve d'Ascq, France c Sciences Chimiques de Rennes, France d LISA, CNRS/Universités Paris Est et Denis Diderot, Créteil, France

Why are we interested in deuterated species? A large number of unidentified lines in the ISM may be due to partially deuterated species. Measuring partially deuterated species provides astronomers with a tool to measure the [D]/[H] ratio. In this talk the microwave spectrum of the partially deuterated species of dimethyl ether CH 2 DOCH 3 will be investigated theoretically.

Outline • Torsional energy levels of the normal species • Torsional energy levels of the deuterated species – Torsional Hamiltonian – Potential energy surface – Torsional functions – Nature of the torsional energy levels • Microwave spectrum of the deuterated species

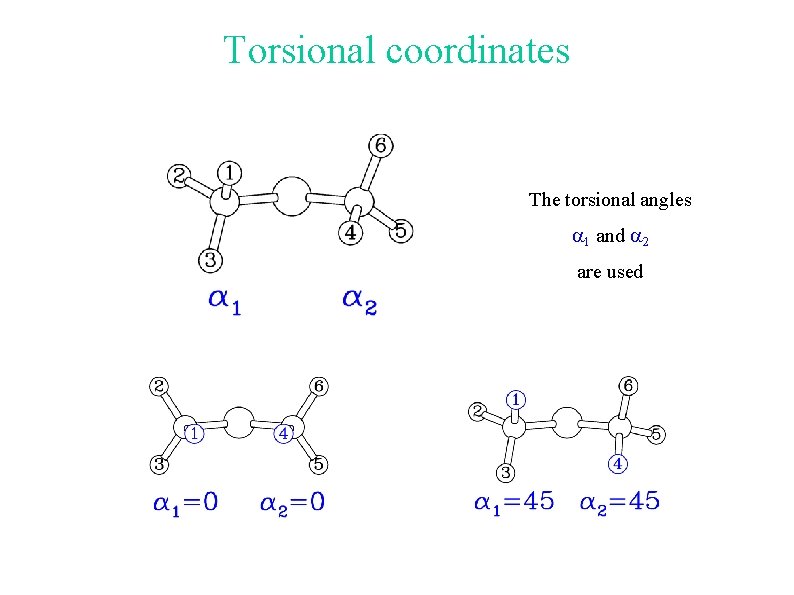
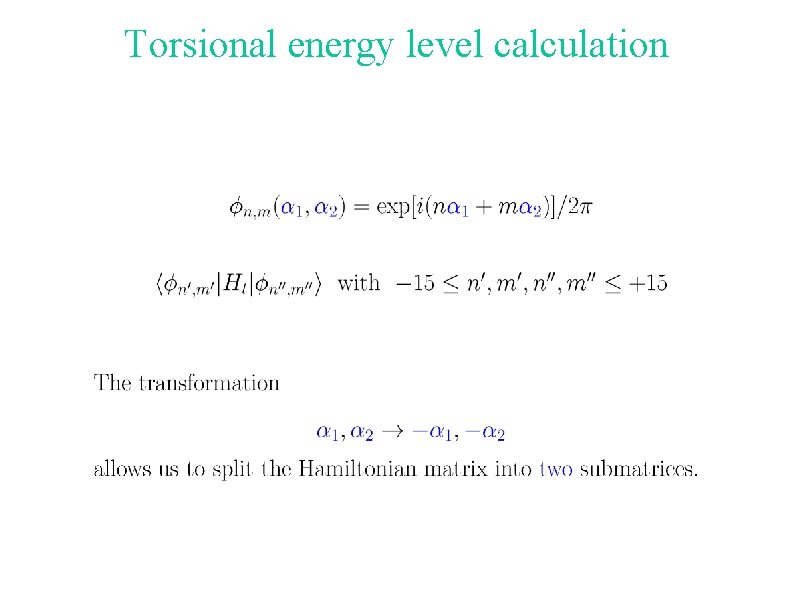
Torsional coordinates The torsional angles a 1 and a 2 are used

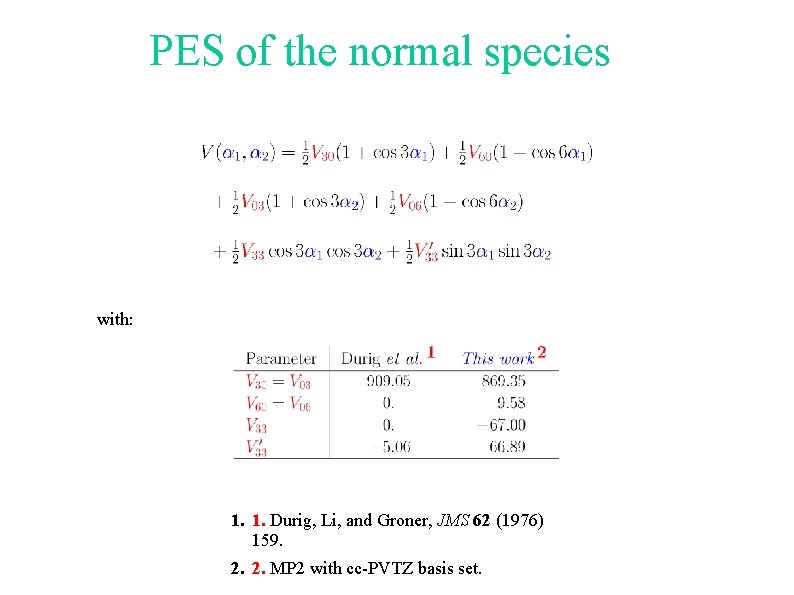
PES of the normal species with: 1. 1. Durig, Li, and Groner, JMS 62 (1976) 159. 2. 2. MP 2 with cc-PVTZ basis set.


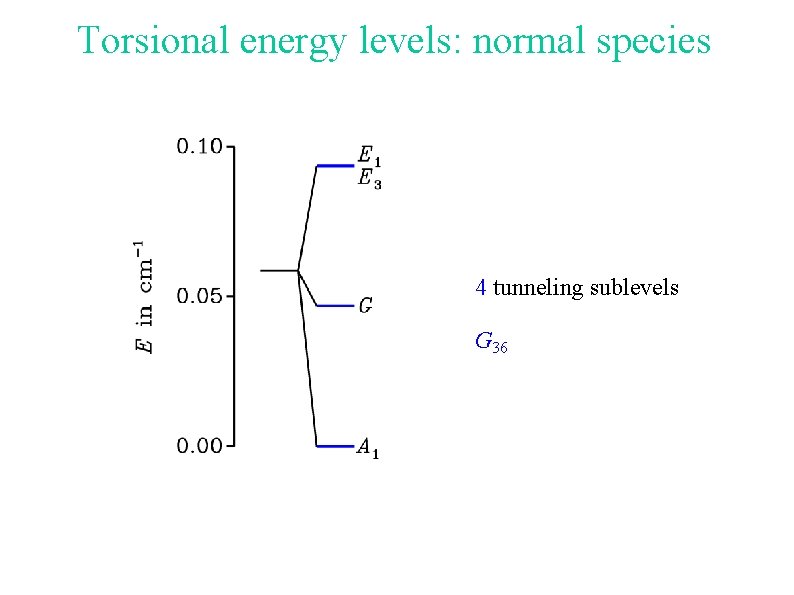
Torsional energy levels: normal species 4 tunneling sublevels G 36

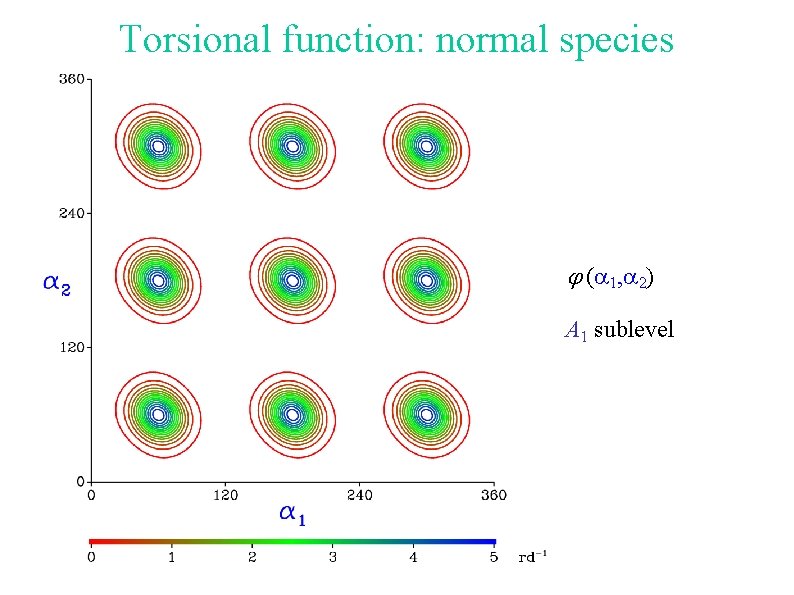
Torsional function: normal species (a 1, a 2) A 1 sublevel

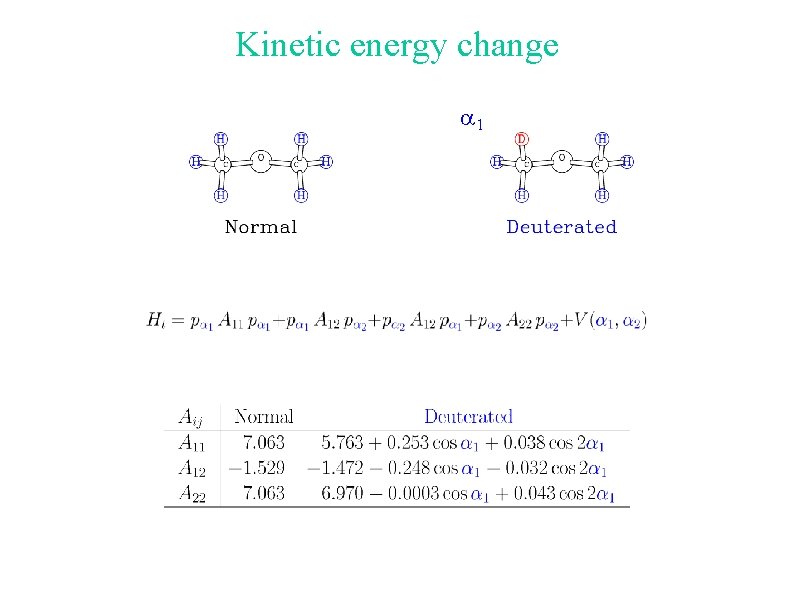
What happens when the molecule is deuterated? The kinetic energy part of the torsional Hamiltonian is modified because of the mass change. The effective potential energy function for the torsion is changed due to zero-point energy effects.

Kinetic energy change a 1

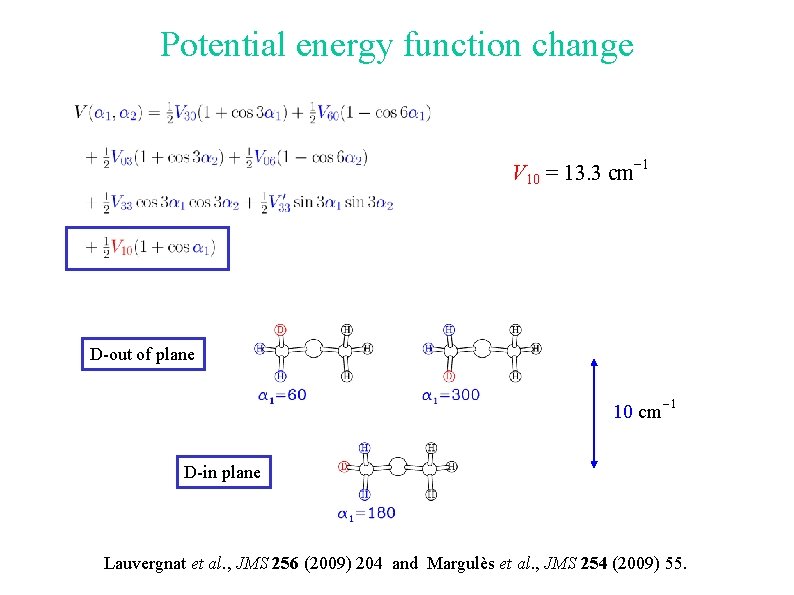
Potential energy function change V 10 = 13. 3 cm-1 D-out of plane 10 cm-1 D-in plane Lauvergnat et al. , JMS 256 (2009) 204 and Margulès et al. , JMS 254 (2009) 55.

Torsional energy level calculation

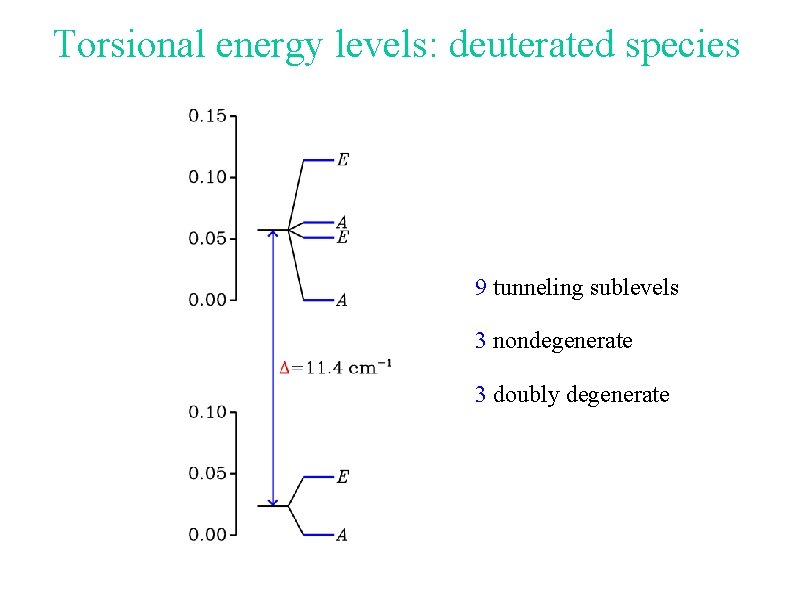
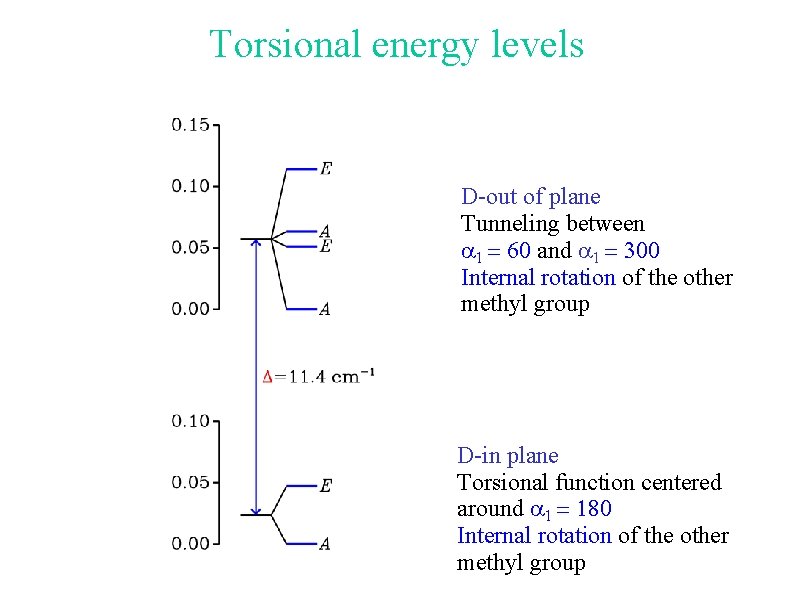
Torsional energy levels: deuterated species 9 tunneling sublevels 3 nondegenerate 3 doubly degenerate

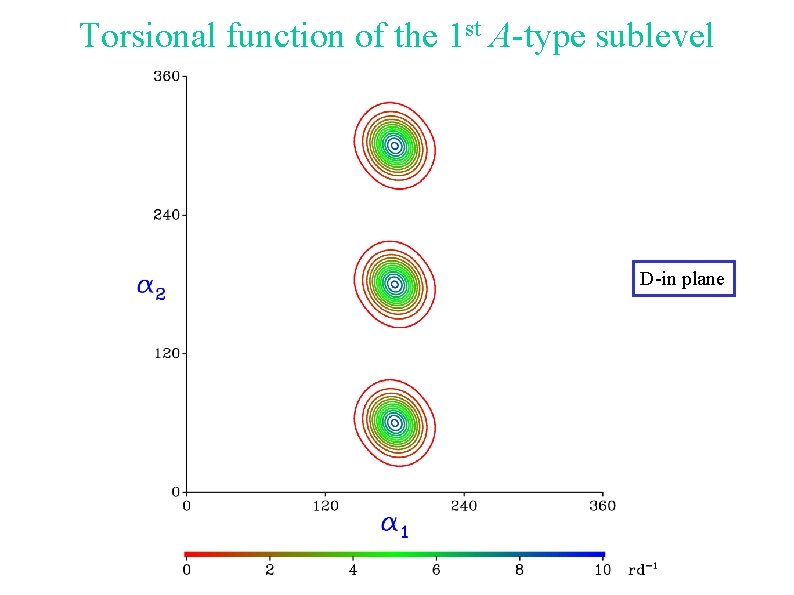
Torsional function of the 1 st A-type sublevel D-in plane

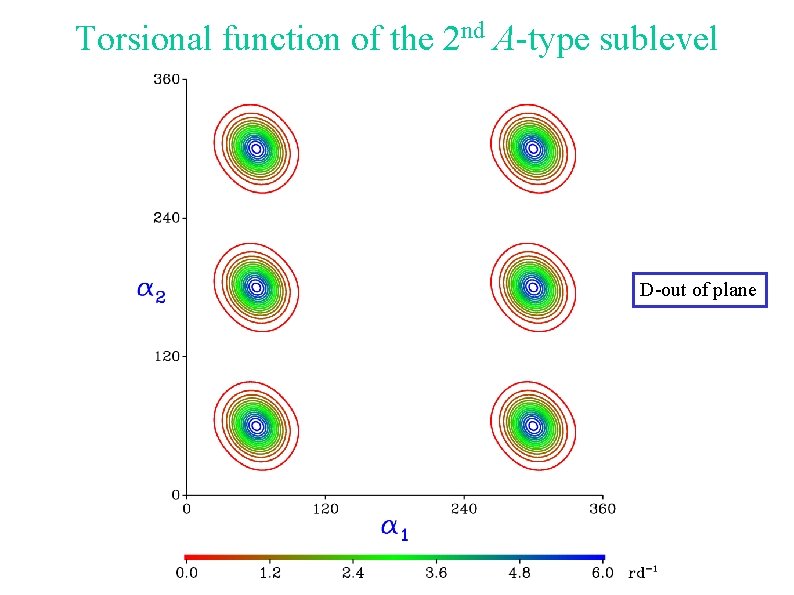
Torsional function of the 2 nd A-type sublevel D-out of plane

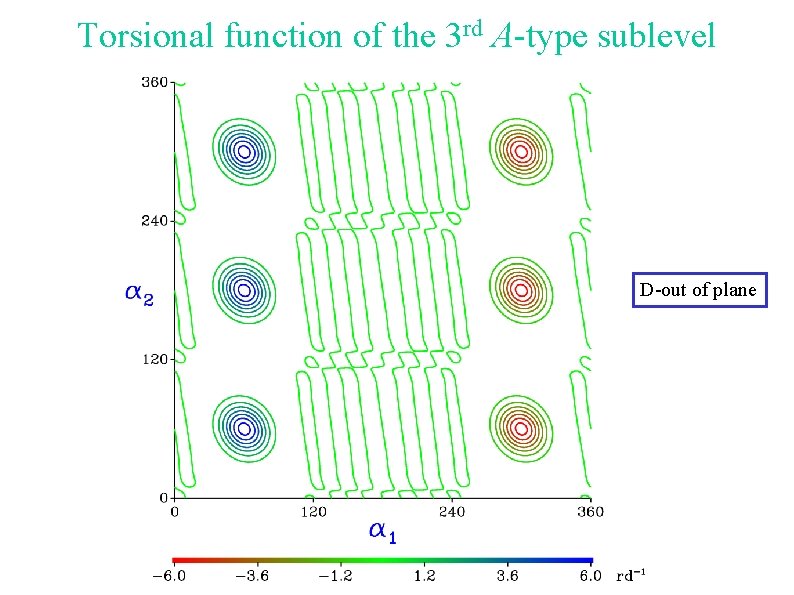
Torsional function of the 3 rd A-type sublevel D-out of plane

Torsional energy levels D-out of plane Tunneling between a 1 = 60 and a 1 = 300 Internal rotation of the other methyl group D-in plane Torsional function centered around a 1 = 180 Internal rotation of the other methyl group

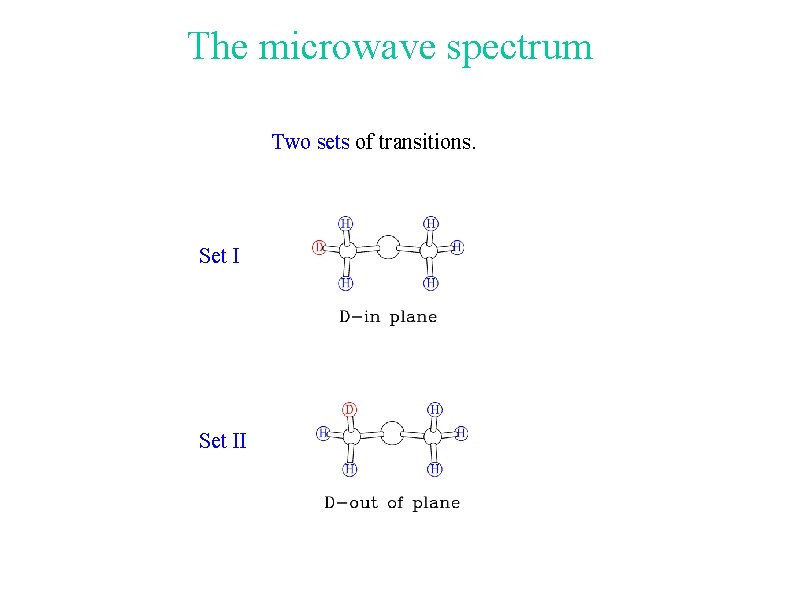
The microwave spectrum Two sets of transitions. Set II

The next steps Overall rotation will be taken into account. Rotational dependence of the various tunneling splittings will be determined using the water dimer formalism. 1 The energy difference D between the two sublevel sets should be calculated accurately. We can begin analyzing the microwave spectrum. 1. Hougen, JMS 114 (1985) 395 and Coudert and Hougen, JMS 139 (1990) 259.
 Cryostat procedure
Cryostat procedure Line spectrum and continuous spectrum difference
Line spectrum and continuous spectrum difference Absortpion
Absortpion Mountain lion keystone species
Mountain lion keystone species Partially filled capacitor
Partially filled capacitor Two pipe drainage system
Two pipe drainage system That assembly does not allow partially trusted callers
That assembly does not allow partially trusted callers B-tree definition
B-tree definition Partially ordered tree
Partially ordered tree Does osmosis require a partially permeable membrane
Does osmosis require a partially permeable membrane Veteork
Veteork Traffic patterns interior design
Traffic patterns interior design Substages of sensorimotor
Substages of sensorimotor Partially filled capacitor
Partially filled capacitor What does partially permeable mean
What does partially permeable mean Partially decayed plant matter found in bogs
Partially decayed plant matter found in bogs Partially concealed
Partially concealed Eco friendly partially addressed mail
Eco friendly partially addressed mail Partially impregnated and surface coated polymer concrete
Partially impregnated and surface coated polymer concrete Bi-rad 4 suspicious abnormality
Bi-rad 4 suspicious abnormality