The Microwave Spectrum of the Mono Deuterated Species



- Slides: 16

The Microwave Spectrum of the Mono Deuterated Species of Methyl Formate HCOOCH 2 D L. H. Coudert, a L. Margulès, b G. Wlodarczak, b and J. Demaisonb a. LISA, CNRS/Paris 12 University, Créteil, France b. Ph. LAM, CNRS/Lille I University, Villeneuve d’Ascq, France

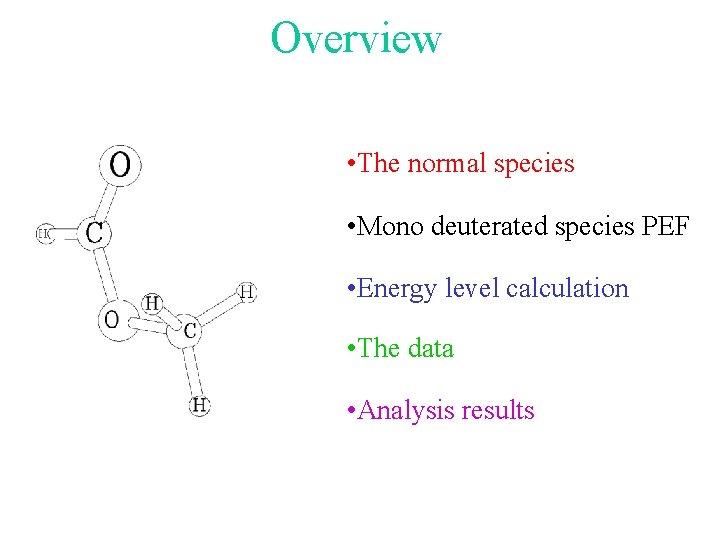
Overview • The normal species • Mono deuterated species PEF • Energy level calculation • The data • Analysis results

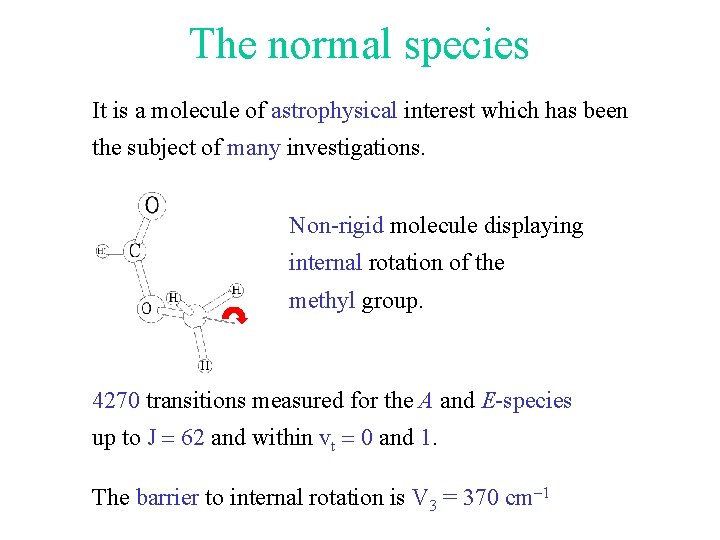
The normal species It is a molecule of astrophysical interest which has been the subject of many investigations. Non-rigid molecule displaying internal rotation of the methyl group. 4270 transitions measured for the A and E-species up to J = 62 and within vt = 0 and 1. The barrier to internal rotation is V 3 = 370 cm-1

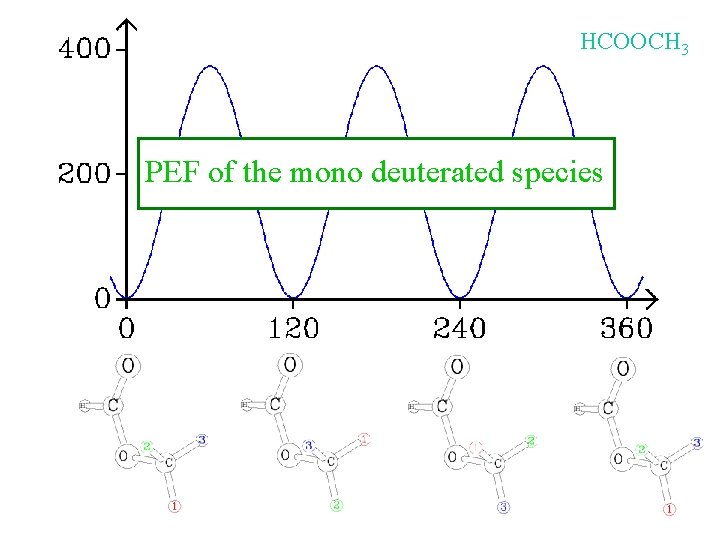
HCOOCH 3 PEF of the mono deuterated species

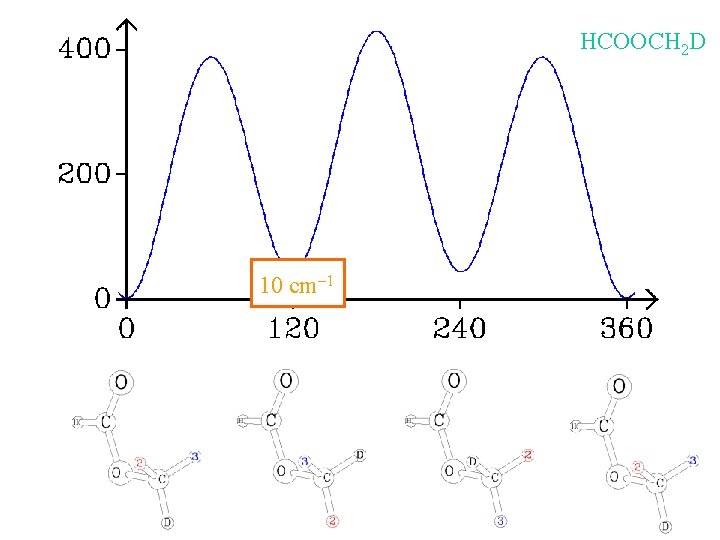
HCOOCH 2 D 10 cm-1

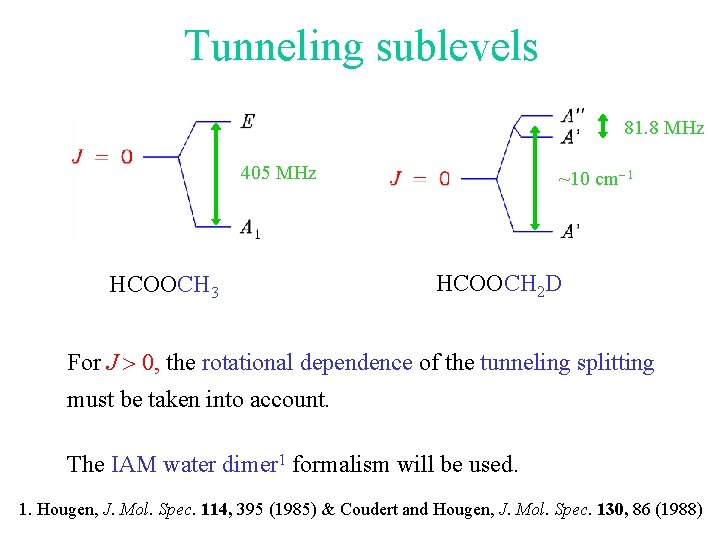
Tunneling sublevels 81. 8 MHz 405 MHz HCOOCH 3 ~10 cm-1 HCOOCH 2 D For J > 0, the rotational dependence of the tunneling splitting must be taken into account. The IAM water dimer 1 formalism will be used. 1. Hougen, J. Mol. Spec. 114, 395 (1985) & Coudert and Hougen, J. Mol. Spec. 130, 86 (1988)

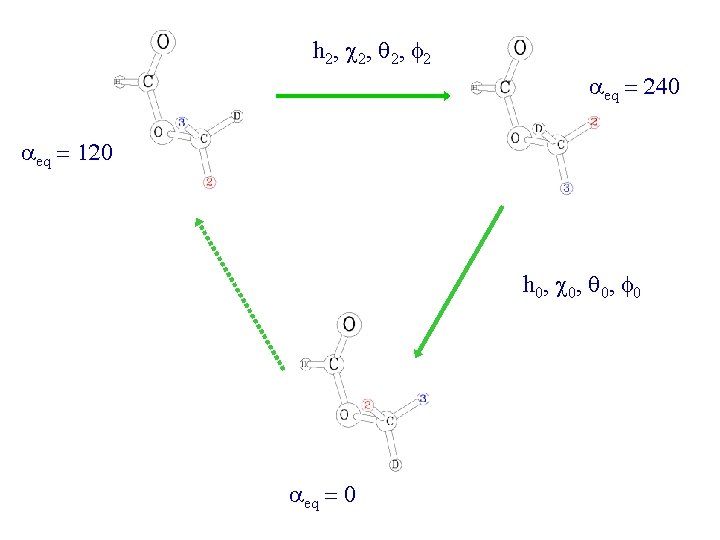
h 2, c 2, q 2, f 2 aeq = 240 aeq = 120 h 0, c 0, q 0, f 0 aeq = 0

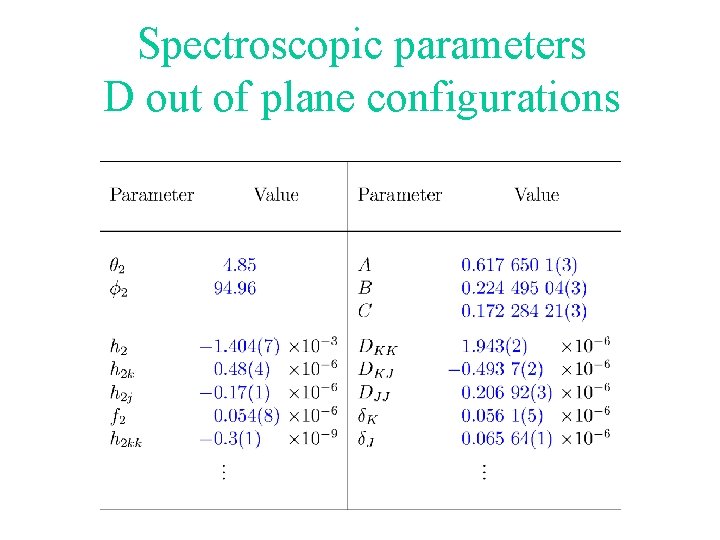
Spectroscopic parameters Parameters Number Relation h 2, c 2, q 2, f 2 3 c 2 = f 2 + p h 0, c 0, q 0, f 0 0 not used A, B, C 3 D in plane A, B, C 3 D out of plane 9 zeroth order parameters

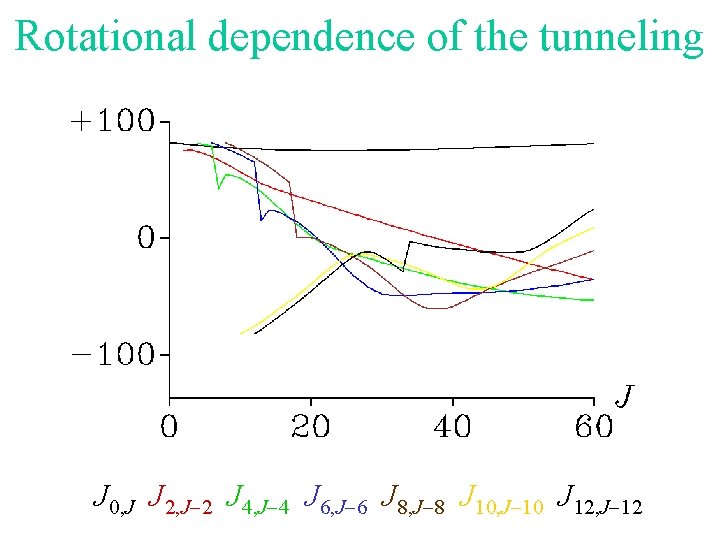
Rotational dependence of the tunneling J 0, J J 2, J-2 J 4, J-4 J 6, J-6 J 8, J-8 J 10, J-10 J 12, J-12

Rotational dependence of the tunneling J 1, J J 3, J-2 J 5, J-4 J 7, J-6 J 9, J-8 J 11, J-10 J 13, J-12

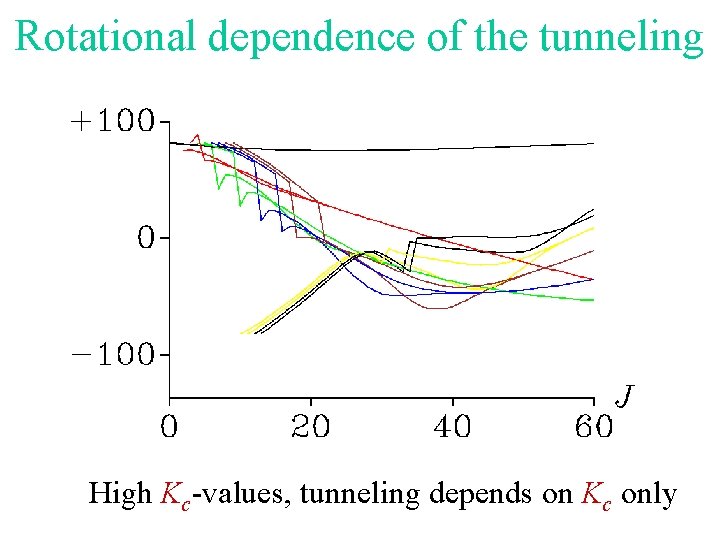
Rotational dependence of the tunneling High Kc-values, tunneling depends on Kc only

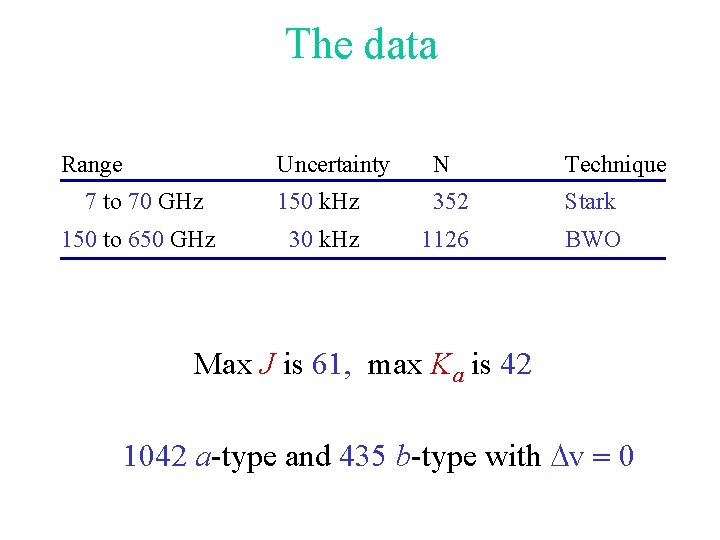
The data Range Uncertainty N Technique 7 to 70 GHz 150 k. Hz 352 Stark 150 to 650 GHz 30 k. Hz 1126 BWO Max J is 61, max Ka is 42 1042 a-type and 435 b-type with Dv = 0

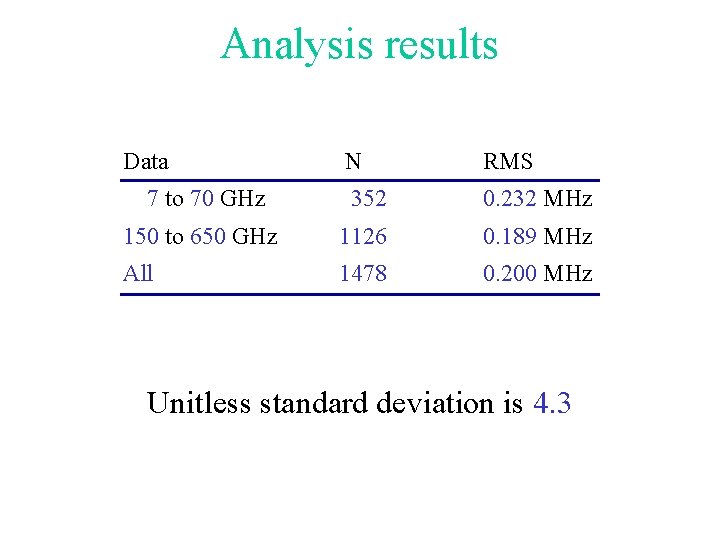
Analysis results Data N RMS 7 to 70 GHz 352 0. 232 MHz 150 to 650 GHz 1126 0. 189 MHz All 1478 0. 200 MHz Unitless standard deviation is 4. 3

Spectroscopic parameters D out of plane configurations

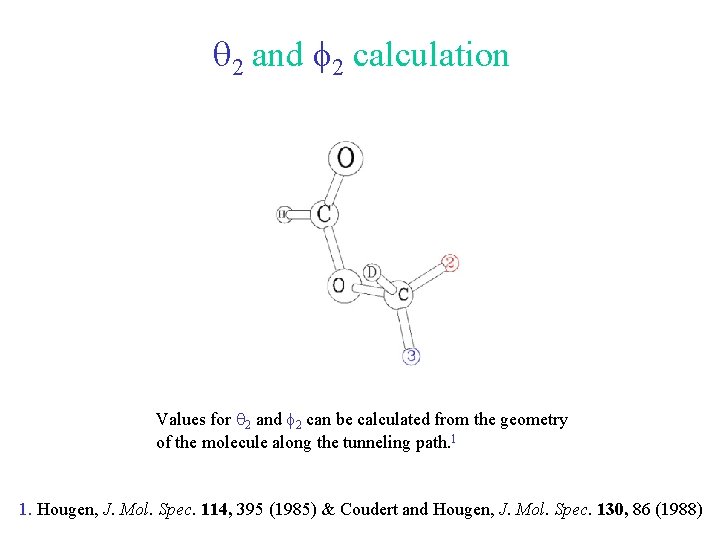
q 2 and f 2 calculation Values for q 2 and f 2 can be calculated from the geometry of the molecule along the tunneling path. 1 1. Hougen, J. Mol. Spec. 114, 395 (1985) & Coudert and Hougen, J. Mol. Spec. 130, 86 (1988)

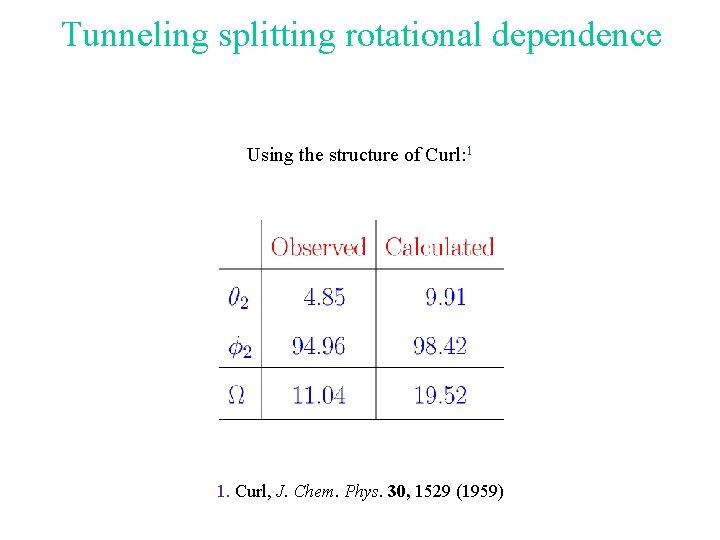
Tunneling splitting rotational dependence Using the structure of Curl: 1 1. Curl, J. Chem. Phys. 30, 1529 (1959)
 Morgan balabanoff
Morgan balabanoff D orbital shape
D orbital shape Absorption spectrum vs emission spectrum
Absorption spectrum vs emission spectrum Species
Species Mono
Mono Polymers are
Polymers are Prefix meaning mono
Prefix meaning mono Nama senyawa si
Nama senyawa si Nh3 covalent compound name
Nh3 covalent compound name çok atomlu iyonlar tablosu
çok atomlu iyonlar tablosu Mono means
Mono means Mono di tri tetra penta hexa
Mono di tri tetra penta hexa Monoalphabetic substitution ciphers
Monoalphabetic substitution ciphers Monofilament extruder
Monofilament extruder Monorhyme poems
Monorhyme poems El mono dorado
El mono dorado Cuando dios creo al burro perro mono y el hombre
Cuando dios creo al burro perro mono y el hombre