Targeting triglycerides New insights from antisense therapy Prof





- Slides: 18

Targeting triglycerides: New insights from antisense therapy Prof. Daniel Gaudet Universite de Montreal Quebec, Canada

ISIS-APOCIIIRx Interim Analysis: A Phase 2 Study with Monotherapy Treatment of Patients with Severe Hypertriglyceridemia Dr. Daniel Gaudet MD Ph. D Dept of Medicine, Université de Montréal and Scientific Director of the Genome Quebec Biobank August 31, 2013

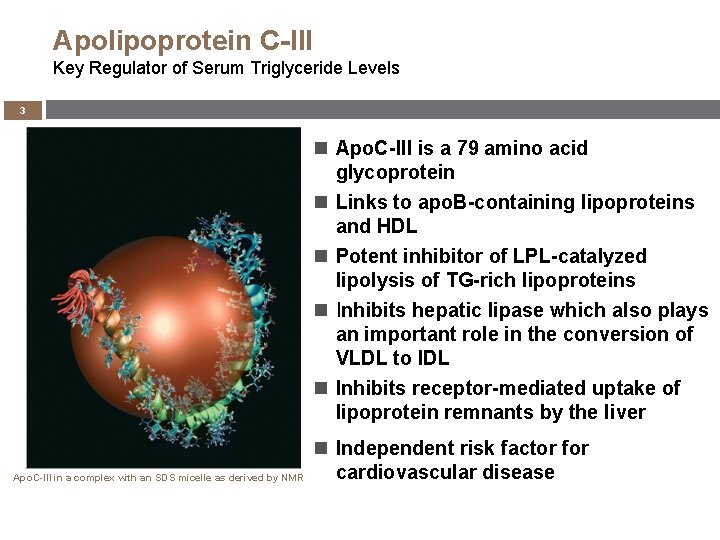
Apolipoprotein C-III Key Regulator of Serum Triglyceride Levels 3 Apo. C-III is a 79 amino acid glycoprotein Links to apo. B-containing lipoproteins and HDL Potent inhibitor of LPL-catalyzed lipolysis of TG-rich lipoproteins Inhibits hepatic lipase which also plays an important role in the conversion of VLDL to IDL Inhibits receptor-mediated uptake of lipoprotein remnants by the liver Apo. C-III in a complex with an SDS micelle as derived by NMR Independent risk factor for cardiovascular disease

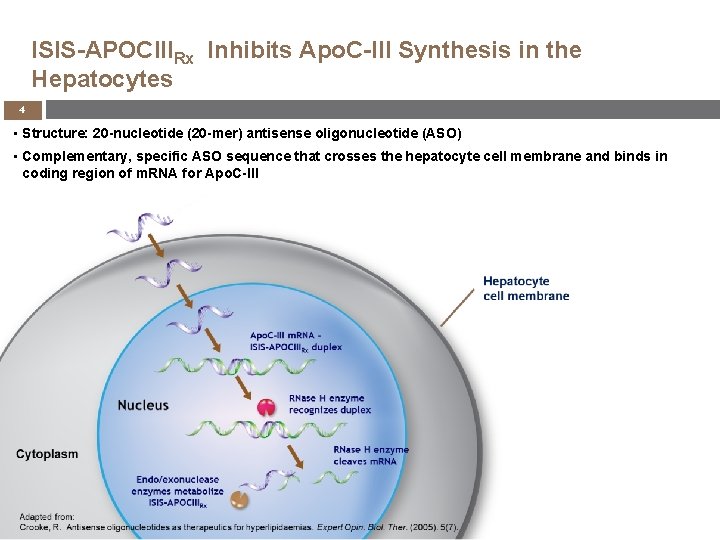
ISIS-APOCIIIRx Inhibits Apo. C-III Synthesis in the Hepatocytes 4 • Structure: 20 -nucleotide (20 -mer) antisense oligonucleotide (ASO) KYNAMRO™ • Complementary, specific ASO sequence that crosses the hepatocyte cell membrane and binds in coding region of m. RNA for Apo. C-III

ISIS-APOCIIIRx Pre-Clinical and Early Phases Studies 5 Pre-clinical results demonstrate that ISIS-APOCIIIRx decreases TG and apo. C-III levels in animal models and improves insulin sensitivity in h. APOC 3 mice Early phase clinical studies suggest that ISIS-APOCIIIRx is safe and well -tolerated with no discontinuations and low incidence of AEs Results of a small randomized, double-blind placebo-controlled phase 2 study evaluating the effect of ISIS-APOCIIIRx in 11 patients with high triglycerides and type 2 diabetes showed improved lipid profile and glucose control Interim results of a Phase 2 Study in 85 patients with moderate to severe hypertriglyceridemia are now available

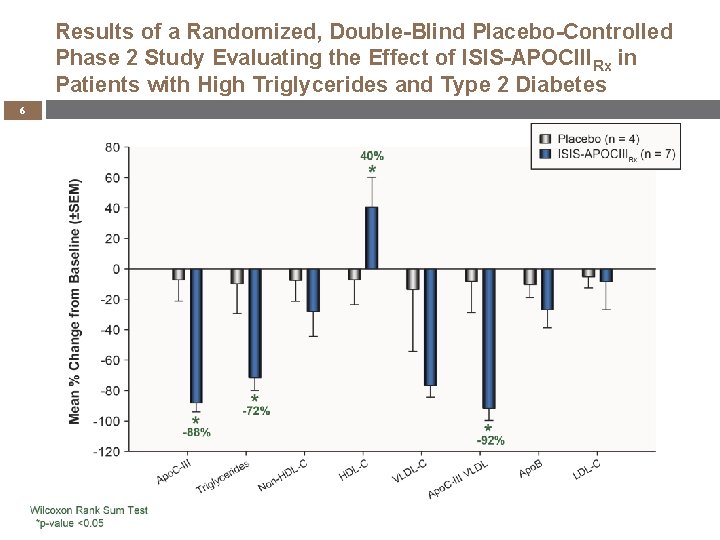
Results of a Randomized, Double-Blind Placebo-Controlled Phase 2 Study Evaluating the Effect of ISIS-APOCIIIRx in Patients with High Triglycerides and Type 2 Diabetes 6

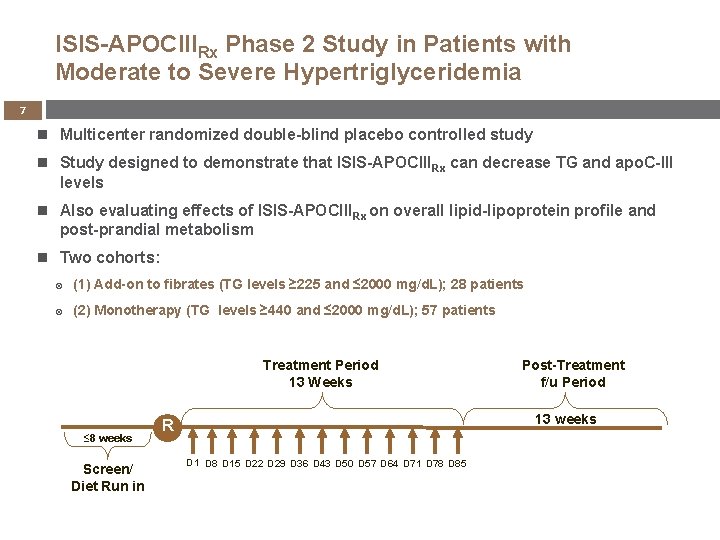
ISIS-APOCIIIRx Phase 2 Study in Patients with Moderate to Severe Hypertriglyceridemia 7 Multicenter randomized double-blind placebo controlled study Study designed to demonstrate that ISIS-APOCIIIRx can decrease TG and apo. C-III levels Also evaluating effects of ISIS-APOCIIIRx on overall lipid-lipoprotein profile and post-prandial metabolism Two cohorts: (1) Add-on to fibrates (TG levels ≥ 225 and ≤ 2000 mg/d. L); 28 patients (2) Monotherapy (TG levels ≥ 440 and ≤ 2000 mg/d. L); 57 patients Treatment Period 13 Weeks ≤ 8 weeks Screen/ Diet Run in Post-Treatment f/u Period 13 weeks R D 1 D 8 D 15 D 22 D 29 D 36 D 43 D 50 D 57 D 64 D 71 D 78 D 85

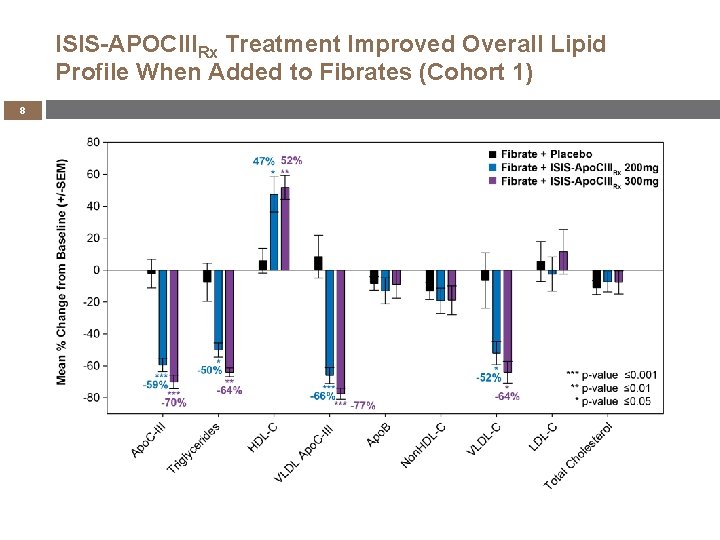
ISIS-APOCIIIRx Treatment Improved Overall Lipid Profile When Added to Fibrates (Cohort 1) 8

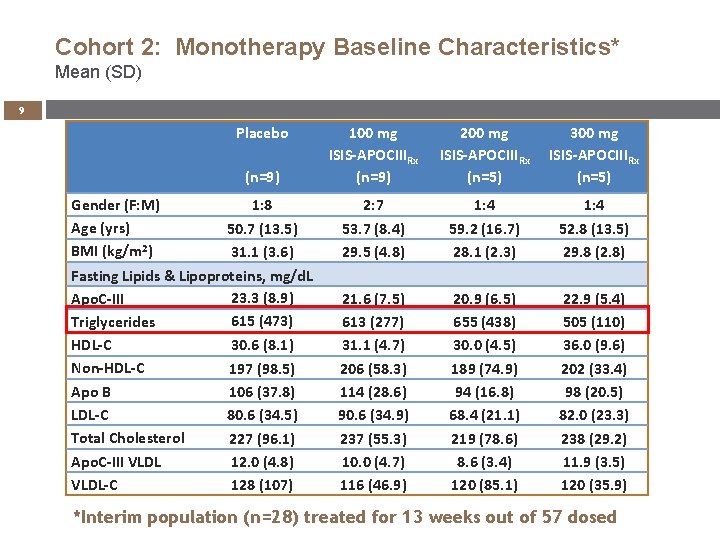
Cohort 2: Monotherapy Baseline Characteristics* Mean (SD) 9 Placebo Gender (F: M) Age (yrs) BMI (kg/m 2) (n=9) 100 mg ISIS-APOCIIIRx (n=9) 200 mg ISIS-APOCIIIRx (n=5) 300 mg ISIS-APOCIIIRx (n=5) 1: 8 2: 7 1: 4 53. 7 (8. 4) 29. 5 (4. 8) 59. 2 (16. 7) 28. 1 (2. 3) 52. 8 (13. 5) 29. 8 (2. 8) 21. 6 (7. 5) 613 (277) 31. 1 (4. 7) 206 (58. 3) 114 (28. 6) 90. 6 (34. 9) 237 (55. 3) 10. 0 (4. 7) 116 (46. 9) 20. 9 (6. 5) 655 (438) 30. 0 (4. 5) 189 (74. 9) 94 (16. 8) 68. 4 (21. 1) 219 (78. 6) 8. 6 (3. 4) 120 (85. 1) 22. 9 (5. 4) 505 (110) 36. 0 (9. 6) 202 (33. 4) 98 (20. 5) 82. 0 (23. 3) 238 (29. 2) 11. 9 (3. 5) 120 (35. 9) 50. 7 (13. 5) 31. 1 (3. 6) Fasting Lipids & Lipoproteins, mg/d. L 23. 3 (8. 9) Apo. C-III 615 (473) Triglycerides HDL-C 30. 6 (8. 1) Non-HDL-C 197 (98. 5) Apo B 106 (37. 8) LDL-C 80. 6 (34. 5) Total Cholesterol 227 (96. 1) Apo. C-III VLDL 12. 0 (4. 8) VLDL-C 128 (107) *Interim population (n=28) treated for 13 weeks out of 57 dosed

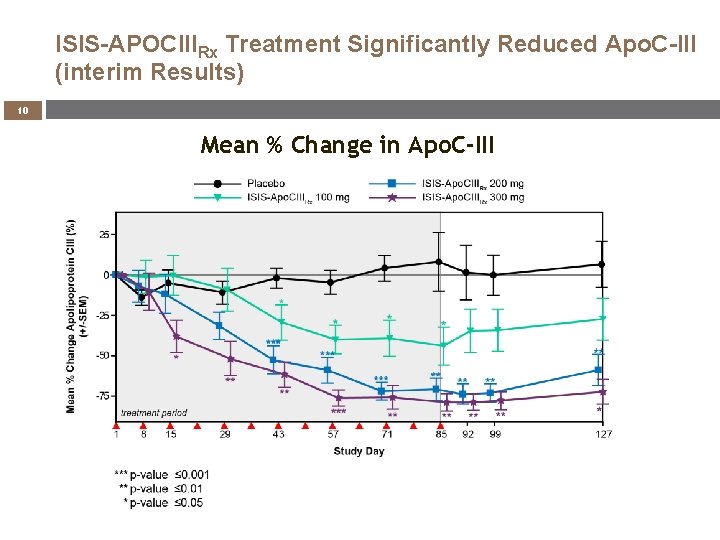
ISIS-APOCIIIRx Treatment Significantly Reduced Apo. C-III (interim Results) 10 Mean % Change in Apo. C-III

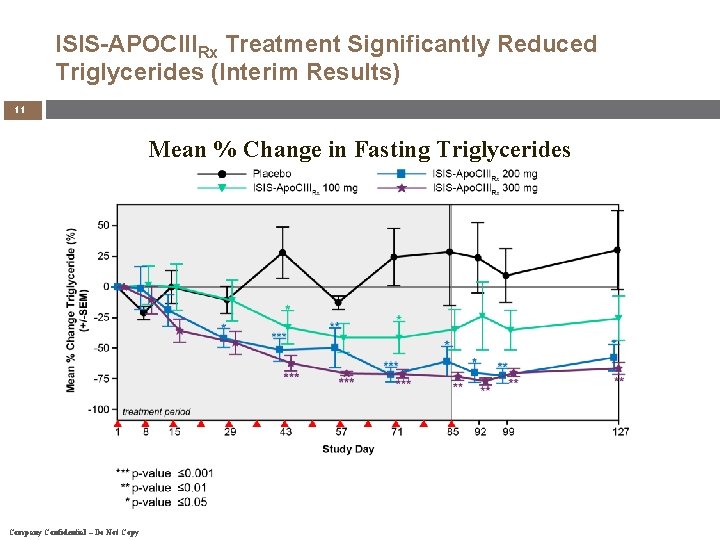
ISIS-APOCIIIRx Treatment Significantly Reduced Triglycerides (Interim Results) 11 Mean % Change in Fasting Triglycerides Company Confidential – Do Not Copy

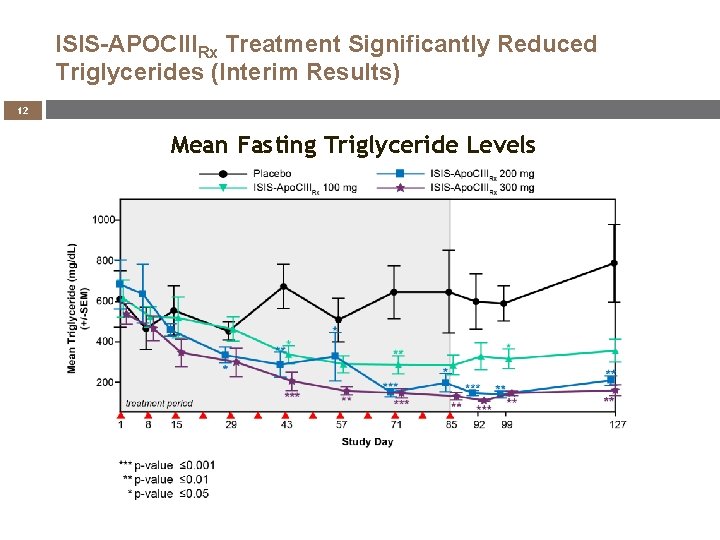
ISIS-APOCIIIRx Treatment Significantly Reduced Triglycerides (Interim Results) 12 Mean Fasting Triglyceride Levels

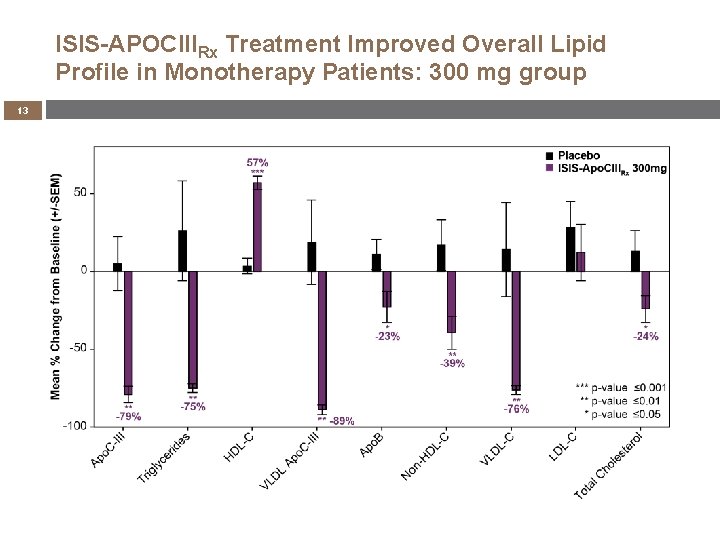
ISIS-APOCIIIRx Treatment Improved Overall Lipid Profile in Monotherapy Patients: 300 mg group 13

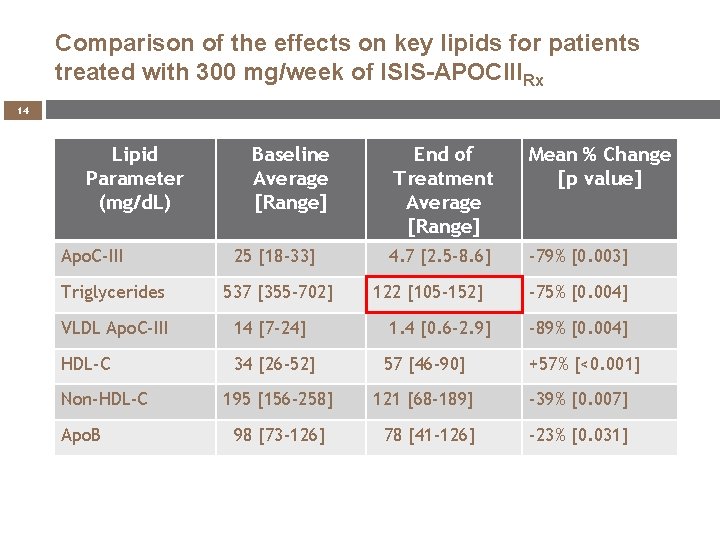
Comparison of the effects on key lipids for patients treated with 300 mg/week of ISIS-APOCIIIRx 14 Lipid Parameter (mg/d. L) Apo. C-III Triglycerides Baseline Average [Range] 25 [18 -33] 537 [355 -702] End of Treatment Average [Range] Mean % Change [p value] 4. 7 [2. 5 -8. 6] -79% [0. 003] 122 [105 -152] VLDL Apo. C-III 14 [7 -24] HDL-C 34 [26 -52] 57 [46 -90] 195 [156 -258] 121 [68 -189] -39% [0. 007] 78 [41 -126] -23% [0. 031] Non-HDL-C Apo. B 98 [73 -126] 1. 4 [0. 6 -2. 9] -75% [0. 004] -89% [0. 004] +57% [<0. 001]

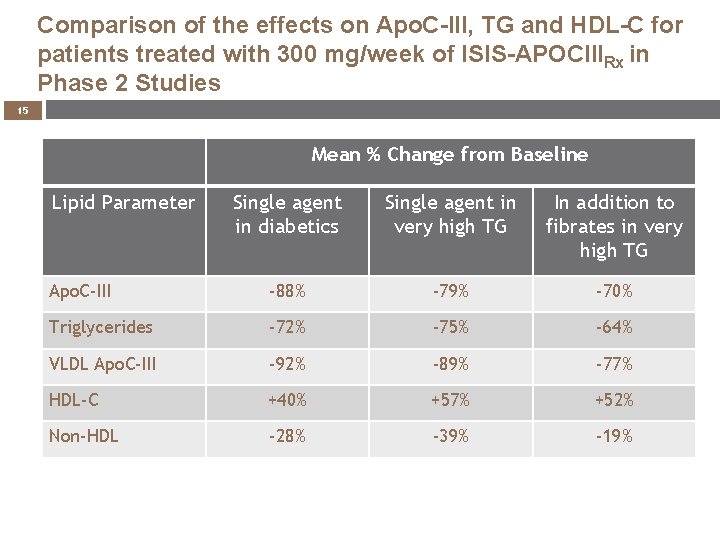
Comparison of the effects on Apo. C-III, TG and HDL-C for patients treated with 300 mg/week of ISIS-APOCIIIRx in Phase 2 Studies 15 Mean % Change from Baseline Lipid Parameter Single agent in diabetics Single agent in very high TG In addition to fibrates in very high TG Apo. C-III -88% -79% -70% Triglycerides -72% -75% -64% VLDL Apo. C-III -92% -89% -77% HDL-C +40% +57% +52% Non-HDL -28% -39% -19%

Safety Summary ISIS-APOCIIIRx Monotherapy in Patients with Severe Hypertriglyceridemia 16 Safety No related SAEs or significant AEs No treatment related liver enzyme elevations No abnormalities in renal function No clinically meaningful changes in other laboratory values Tolerability No flu-like reactions Infrequent and predominantly mild injection site reactions

ISIS-APOCIIIRx Phase 2 Summary 17 Significantly reduced triglycerides and apo. C-III 86% of patients treated with 300 mg ISIS-APOCIIIRx in both studies achieved TG levels <150 mg/d. L Improved lipid profile Decreased triglycerides, total- and VLDL-Apo. CIII, and non-HDL, and increased HDL-C Effective as a single agent or when used in combination with fibrates Equally effective irrespective of the triglyceride levels at entry Generally safe and well tolerated

Acknowledgements 18 Ecogene-21 Clinical Research Center; Chicoutimi, Quebec Clinical Trial Management Group; Greenville/Raleigh, North Carolina Isis Pharmaceuticals; Carlsbad, California The patients