SYSTEMIC ASSESSMENT AS A VEHICLE TO ASSESS STUDENT

![INTRODUCTION -Systemic Assessment [SA] has been shown to be highly effective new vehicle in; INTRODUCTION -Systemic Assessment [SA] has been shown to be highly effective new vehicle in;](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-2.jpg)
![Why Systemic Assessment (SA) ? [1] It measures the cognitive structure from the cumulative Why Systemic Assessment (SA) ? [1] It measures the cognitive structure from the cumulative](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-3.jpg)
![[ 5 ] Assess the students in a wide range of Concepts in the [ 5 ] Assess the students in a wide range of Concepts in the](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-4.jpg)

![Types Systemic Assessment Questions: [SAQ, s] 1 - Systemic Multiple Choice Questions [SMCQ, s]. Types Systemic Assessment Questions: [SAQ, s] 1 - Systemic Multiple Choice Questions [SMCQ, s].](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-6.jpg)

![Q 3. The systemic diagram represents the following reactions sequence. [Substitution –Substitution- Elimination –Addition-] Q 3. The systemic diagram represents the following reactions sequence. [Substitution –Substitution- Elimination –Addition-]](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-10.jpg)
![Type-2: Systemic True False Questions [STFQ, s] are well suited for testing student comprehension, Type-2: Systemic True False Questions [STFQ, s] are well suited for testing student comprehension,](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-11.jpg)
![Type. 3 : SYSTEMIC MATCHING QUESTIONS: [SMQ, s] Form I: Matching on Trigonal Systemic Type. 3 : SYSTEMIC MATCHING QUESTIONS: [SMQ, s] Form I: Matching on Trigonal Systemic](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-16.jpg)
![Type-4: Systemic Sequencing Question [SSQ, s] Measure the student's ability to find the relationship Type-4: Systemic Sequencing Question [SSQ, s] Measure the student's ability to find the relationship](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-20.jpg)
![Type-5: Systemic Synthesis Questions [SSyn. Q, s] SSyn. Q, s means synthesize systemic relations. Type-5: Systemic Synthesis Questions [SSyn. Q, s] SSyn. Q, s means synthesize systemic relations.](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-24.jpg)
![Type-6: Systemic Analysis Questions [SSAn. Q, s] Requires the Student to Analyze the given Type-6: Systemic Analysis Questions [SSAn. Q, s] Requires the Student to Analyze the given](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-27.jpg)



- Slides: 32

SYSTEMIC ASSESSMENT AS A VEHICLE TO ASSESS STUDENT ACHEIVEMENTS IN CHEMISTRY By Prof. FAHMY A. F. M. E-mail: afmfahmy 42@gmail. com ; web site: www. satlcentral. com 1 st ACRICE –W-1 Dec. 2013
![INTRODUCTION Systemic Assessment SA has been shown to be highly effective new vehicle in INTRODUCTION -Systemic Assessment [SA] has been shown to be highly effective new vehicle in;](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-2.jpg)
INTRODUCTION -Systemic Assessment [SA] has been shown to be highly effective new vehicle in; -Raising the level of students academic achievements. -Increasing equity of students learning outcomes. -Improving students’ ability to learn by enhancing the process of teaching and learning. -Involving the student as an active candidate in this process.
![Why Systemic Assessment SA 1 It measures the cognitive structure from the cumulative Why Systemic Assessment (SA) ? [1] It measures the cognitive structure from the cumulative](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-3.jpg)
Why Systemic Assessment (SA) ? [1] It measures the cognitive structure from the cumulative (quantitative) to the interactive and tuned (qualitative). [2] Assess students higher-order thinking skills in which students are required to analyze, synthesize, and evaluate. [3] Measures the students’ ability to correlate between concepts. [4] Enables the students to discover new relation between concepts.
![5 Assess the students in a wide range of Concepts in the [ 5 ] Assess the students in a wide range of Concepts in the](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-4.jpg)
[ 5 ] Assess the students in a wide range of Concepts in the course unites. [ 6 ] Measures the systemic ILO, s, beside separate Linear ILO, s. [ 7 ] Develop the ability to think systemically, critically and creatively, and Solve problems. [ 8 ] Being objective, realistic and valid. [ 9 ] Very easily scored.

Goal of Systemic Assessment: - The main Goal of SA is to enhance, support and improve both teaching and learning processes via: 1] Enable students to make feedback and feed forward during their study of any course materials. [2]Help students in making maximum connections between Chemistry concepts, compounds, and reactions. [3] Enable students to achieve the highest standards they able. Help teachers to use evidence of student learning to assess student achievement against goals and standards of the courses and programs. .
![Types Systemic Assessment Questions SAQ s 1 Systemic Multiple Choice Questions SMCQ s Types Systemic Assessment Questions: [SAQ, s] 1 - Systemic Multiple Choice Questions [SMCQ, s].](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-6.jpg)
Types Systemic Assessment Questions: [SAQ, s] 1 - Systemic Multiple Choice Questions [SMCQ, s]. 2 -Systemic True, False Questions [STFQ, s]. 3 -Systemic Matching Questions [SMQ, s]. 4 -Systemic Sequencing Questions [SSQ, s]. 5 -Systemic Synthesis Questions [SSyn. Q, s]. 6 - Systemic Analysis Questions [SAn. Q, s].

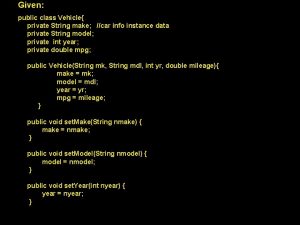
Type-1: Systemic Multiple Choice Questions (SMCQs) MCQs are the traditional choose one from a list of possible answers. However (SMCQs) are choose of one systemic from a list of possible systemics. Each systemic represents at least three chemical relations.

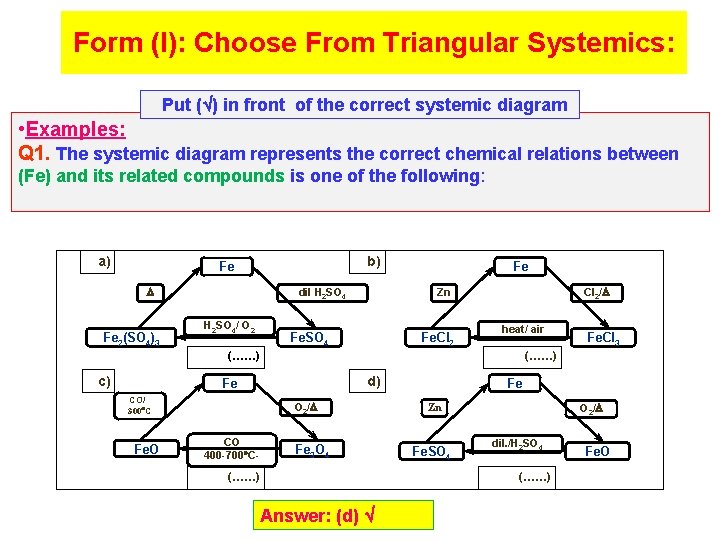
Form (I): Choose From Triangular Systemics: Put ( ) in front of the correct systemic diagram • Examples: Q 1. The systemic diagram represents the correct chemical relations between (Fe) and its related compounds is one of the following: a) b) Fe 2(SO 4)3 dil H 2 SO 4/ O 2 Fe Cl 2/ Zn Fe. SO 4 Fe. Cl 2 heat/ air (……) c) (……) d) Fe CO/ 300 C Fe. O O 2/ CO 400 -700 C- Fe. Cl 3 Fe 3 O 4 (……) Answer: (d) Fe O 2/ Zn Fe. SO 4 dil. /H 2 SO 4 (……) Fe. O

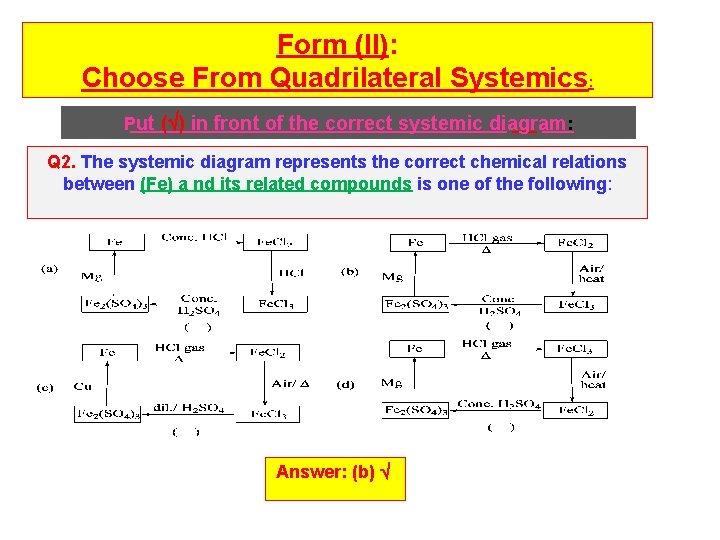
Form (II): Choose From Quadrilateral Systemics: Put ( ) in front of the correct systemic diagram: Q 2. The systemic diagram represents the correct chemical relations between (Fe) a nd its related compounds is one of the following: Answer: (b)
![Q 3 The systemic diagram represents the following reactions sequence Substitution Substitution Elimination Addition Q 3. The systemic diagram represents the following reactions sequence. [Substitution –Substitution- Elimination –Addition-]](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-10.jpg)
Q 3. The systemic diagram represents the following reactions sequence. [Substitution –Substitution- Elimination –Addition-] is one of the following: Answer: (a)
![Type2 Systemic True False Questions STFQ s are well suited for testing student comprehension Type-2: Systemic True False Questions [STFQ, s] are well suited for testing student comprehension,](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-11.jpg)
Type-2: Systemic True False Questions [STFQ, s] are well suited for testing student comprehension, synthesis and analysis, and require a student to assess whether the systemic is true or false. STFQ, s

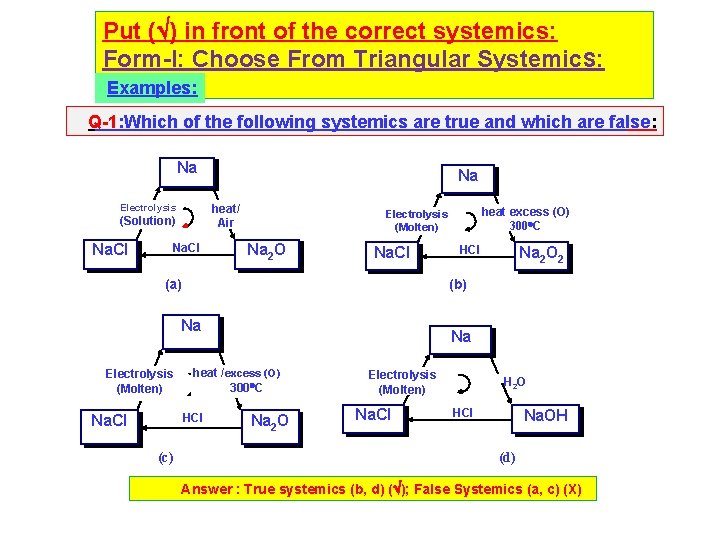
Put ( ) in front of the correct systemics: Form-I: Choose From Triangular Systemic. S: Examples: Q-1: Which of the following systemics are true and which are false : Na Electrolysis heat/ Air (Solution) Na. Cl Na 2 O Na. Cl (a) Na. Cl Na heat /excess (O) 300 C HCl (c) Na 2 O 2 HCl (b) Na Electrolysis (Molten) heat excess (O) 300 C Electrolysis (Molten) Na 2 O Electrolysis (Molten) Na. Cl H 2 O Na. OH HCl (d) Answer : True systemics (b, d) ( ); False Systemics (a, c) (X)

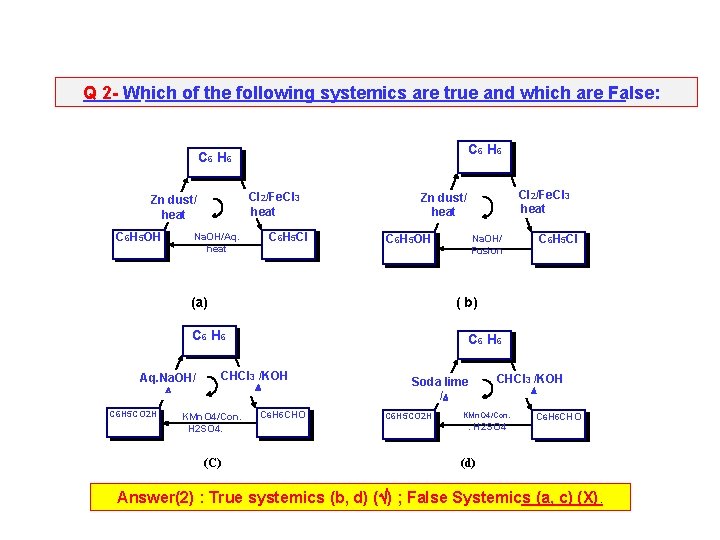
Q 2 - Which of the following systemics are true and which are False: C 6 H 6 Cl 2/Fe. Cl 3 heat Zn dust/ heat C 6 H 5 OH C 6 H 5 Cl Na. OH/Aq. heat C 6 H 5 OH (a) C 6 H 6 CHCl 3 /KOH C 6 H 5 CO 2 H KMn. O 4/Con. H 2 SO 4. (C) C 6 H 5 Cl Na. OH/ Fusion ( b) C 6 H 6 Aq. Na. OH/ Cl 2/Fe. Cl 3 heat Zn dust/ heat C 6 H 5 CHO Soda lime / C 6 H 5 CO 2 H CHCl 3 /KOH KMn. O 4/Con. . H 2 SO 4 C 6 H 5 CHO (d) Answer(2) : True systemics (b, d) ( ) ; False Systemics (a, c) (X).

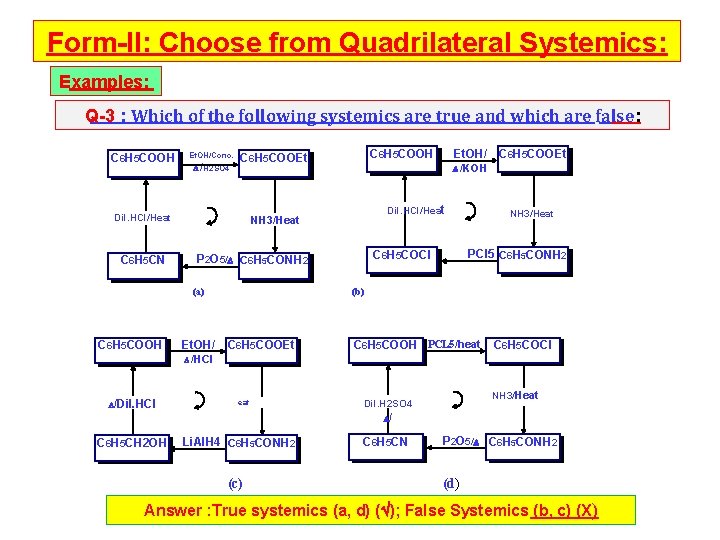
Form-II: Choose from Quadrilateral Systemics: Examples: Q-3 : Which of the following systemics are true and which are false: C 6 H 5 COOH Et. OH/Conc. /H 2 SO 4 Dil. HCl/Heat C 6 H 5 CN Dil. HCl/Heat C 6 H 5 COOEt NH 3/Heat PCl 5 C 6 H 5 CONH 2 C 6 H 5 COCl P 2 O 5/ C 6 H 5 CONH 2 Et. OH/ /KOH NH 3/Heat (a) C 6 H 5 COOH C 6 H 5 COOEt (b) C 6 H 5 COOEt C 6 H 5 COOH PCL 5/heat C 6 H 5 COCl /HCl /Dil. HCl eat NH 3/Heat Dil. H 2 SO 4 / C 6 H 5 CH 2 OH Li. Al. H 4 C 6 H 5 CONH 2 (c) C 6 H 5 CN P 2 O 5/ C 6 H 5 CONH 2 (d) ) Answer : True systemics (a, d) ( ); False Systemics (b, c) (X)

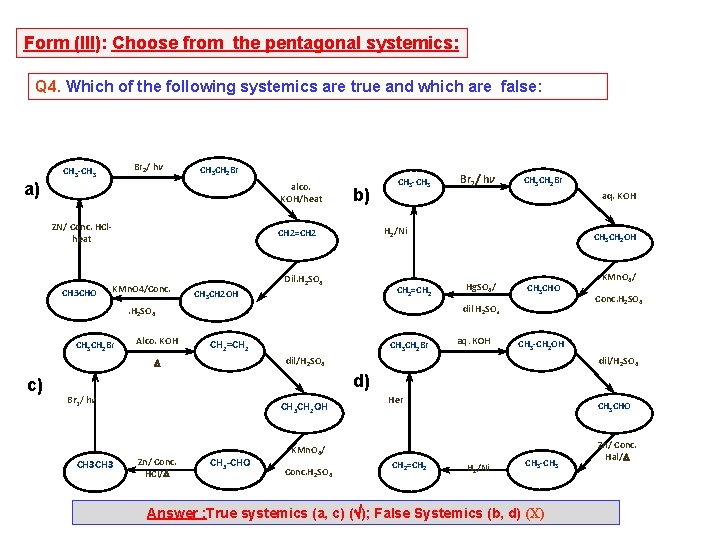
Form (III): Choose from the pentagonal systemics: Q 4. Which of the following systemics are true and which are false: Br 2/ hv CH 3 -CH 3 CH 2 Br a) alco. KOH/heat ZN/ Conc. HClheat CH 3 CHO b) Alco. KOH Dil. H 2 SO 4 CH 2=CH 2 CH 3 CH 2 OH Hg. SO 4/ CH 3 CHO dil H 2 SO 4 CH 2=CH 2 CH 3 CH 2 Br aq. KOH KMn. O 4/ Conc. H 2 SO 4 CH 3 -CH 2 OH dil/H 2 SO 4 c) CH 3 CH 2 Br aq. KOH . H 2 SO 4 CH 3 CH 2 Br Br 2/ hv H 2/Ni CH 2=CH 2 KMn. O 4/Conc. CH 3 -CH 3 dil/H 2 SO 4 d) Br 2/ hv CH 3 CH 2 OH Zn/ Conc. HCl/ CH 3 -CHO Her CH 3 CHO KMn. O 4/ Conc. H 2 SO 4 CH 2=CH 2 H 2/Ni CH 3 -CH 3 Answer : True systemics (a, c) ( ); False Systemics (b, d) (X) Zn/ Conc. Hal/
![Type 3 SYSTEMIC MATCHING QUESTIONS SMQ s Form I Matching on Trigonal Systemic Type. 3 : SYSTEMIC MATCHING QUESTIONS: [SMQ, s] Form I: Matching on Trigonal Systemic](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-16.jpg)
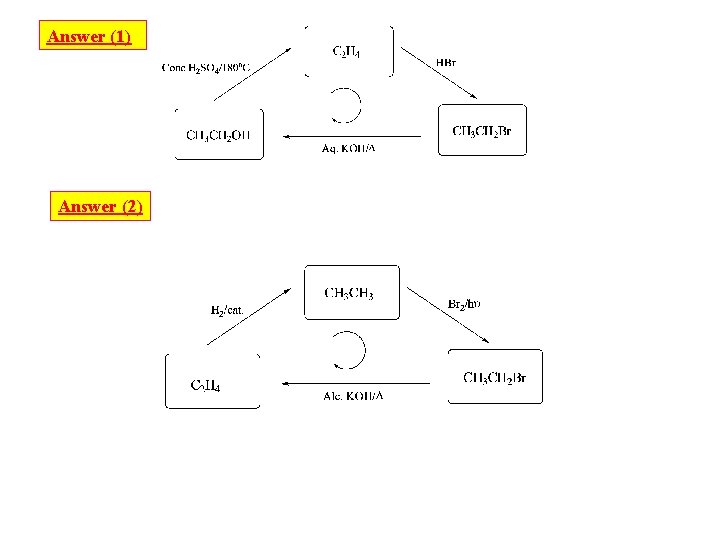
Type. 3 : SYSTEMIC MATCHING QUESTIONS: [SMQ, s] Form I: Matching on Trigonal Systemic Q 1 : Choose aliphatic compounds from column (A) and reaction conditions from column (B) to build the systemic diagram incolumn (C): (A) (C) (B) C 2 H 4 dil. H 2 SO 4 CH 3 Conc H 2 SO 4/180 OC CH 3 CH 2 OH PBr 3 CH 3 CH 2 Br Alc. KOH/∆ Aq. KOH/∆ HBr H 2/cat. Br 2/hν

Answer (1) Answer (2)

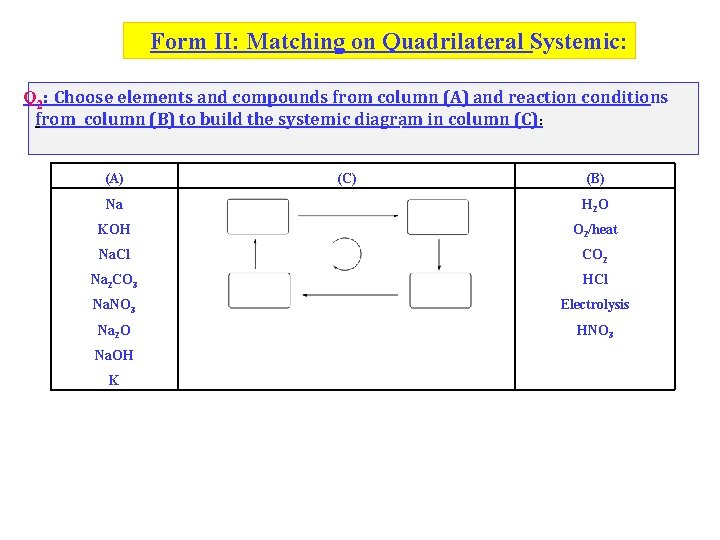
Form II: Matching on Quadrilateral Systemic: Q 2: Choose elements and compounds from column (A) and reaction conditions from column (B) to build the systemic diagram in column (C): (A) (C) (B) Na H 2 O KOH O 2/heat Na. Cl CO 2 Na 2 CO 3 HCl Na. NO 3 Electrolysis Na 2 O HNO 3 Na. OH K

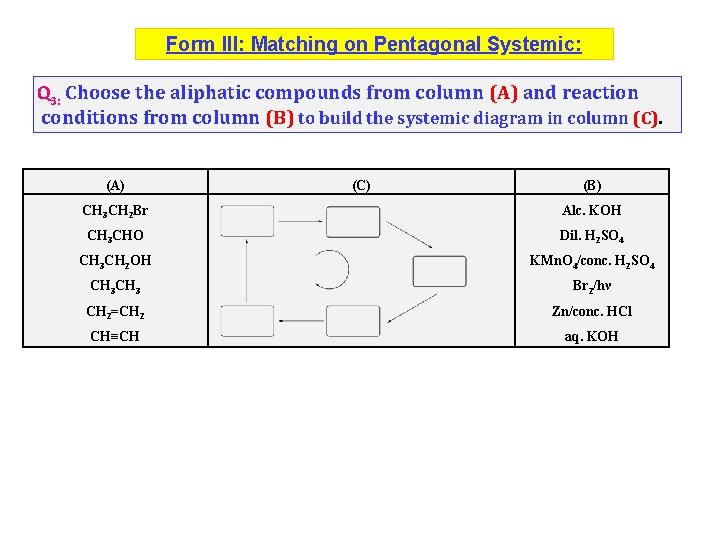
Form III: Matching on Pentagonal Systemic: Q 3: Choose the aliphatic compounds from column (A) and reaction conditions from column (B) to build the systemic diagram in column (C). (A) (C) (B) CH 3 CH 2 Br Alc. KOH CH 3 CHO Dil. H 2 SO 4 CH 3 CH 2 OH KMn. O 4/conc. H 2 SO 4 CH 3 Br 2/hν CH 2=CH 2 Zn/conc. HCl CH≡CH aq. KOH
![Type4 Systemic Sequencing Question SSQ s Measure the students ability to find the relationship Type-4: Systemic Sequencing Question [SSQ, s] Measure the student's ability to find the relationship](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-20.jpg)
Type-4: Systemic Sequencing Question [SSQ, s] Measure the student's ability to find the relationship between a set of similar items, and then arrange them in a given systemic.

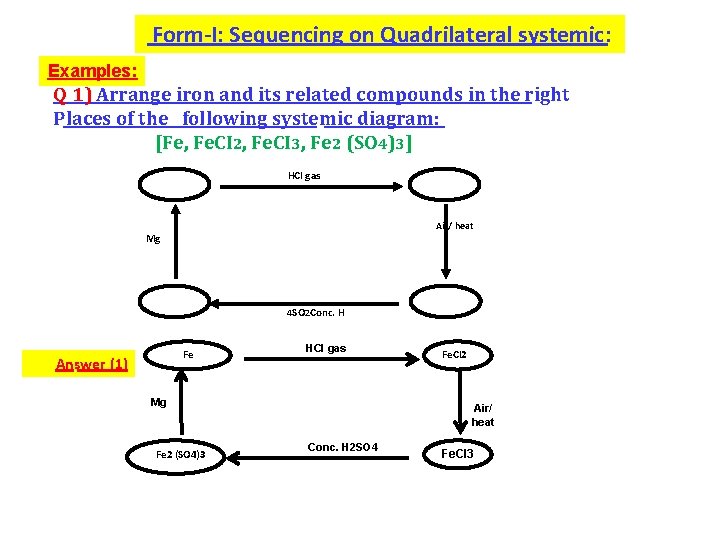
Form-I: Sequencing on Quadrilateral systemic: Examples: Q 1) Arrange iron and its related compounds in the right Places of the following systemic diagram: [Fe, Fe. CI 2, Fe. CI 3, Fe 2 (SO 4)3] HCl gas Air/ heat Mg 4 SO 2 Conc. H Fe Answer (1) HCl gas Mg Fe 2 (SO 4)3 Fe. Cl 2 Air/ heat Conc. H 2 SO 4 Fe. Cl 3

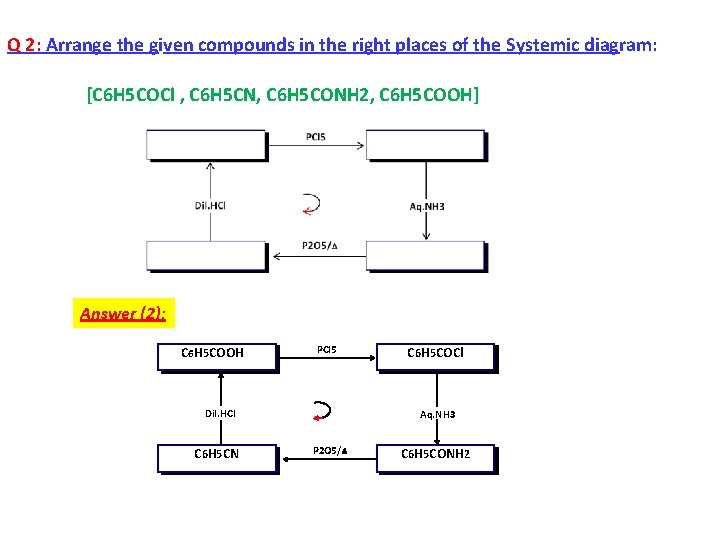
Q 2: Arrange the given compounds in the right places of the Systemic diagram: [C 6 H 5 COCl , C 6 H 5 CN, C 6 H 5 CONH 2, C 6 H 5 COOH] Answer (2): C 6 H 5 COOH PCl 5 Dil. HCl C 6 H 5 CN C 6 H 5 COCl Aq. NH 3 P 2 O 5/ C 6 H 5 CONH 2

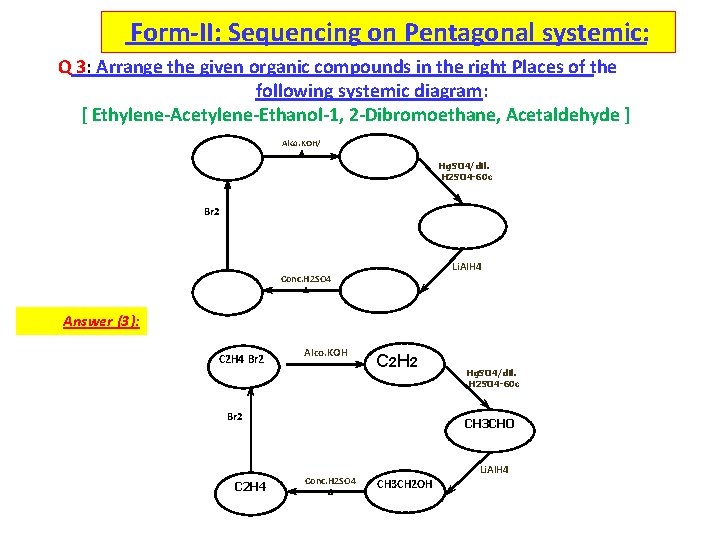
Form-II: Sequencing on Pentagonal systemic: Q 3: Arrange the given organic compounds in the right Places of the following systemic diagram: [ Ethylene-Acetylene-Ethanol-1, 2 -Dibromoethane, Acetaldehyde ] Alco. KOH/ Hg. SO 4/dil. H 2 SO 4 -60 c Br 2 Li. Al. H 4 Conc. H 2 SO 4 Answer (3): C 2 H 4 Br 2 Alco. KOH C 2 H 2 Br 2 C 2 H 4 Hg. SO 4/dil. H 2 SO 4 -60 c CH 3 CHO Conc. H 2 SO 4 Li. Al. H 4 CH 3 CH 2 OH
![Type5 Systemic Synthesis Questions SSyn Q s SSyn Q s means synthesize systemic relations Type-5: Systemic Synthesis Questions [SSyn. Q, s] SSyn. Q, s means synthesize systemic relations.](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-24.jpg)
Type-5: Systemic Synthesis Questions [SSyn. Q, s] SSyn. Q, s means synthesize systemic relations. concepts, facts, Rbetween equires the Student to Pissues, osition concepts or formulas, or events or issues , numbers and their relations in a systemic diagram

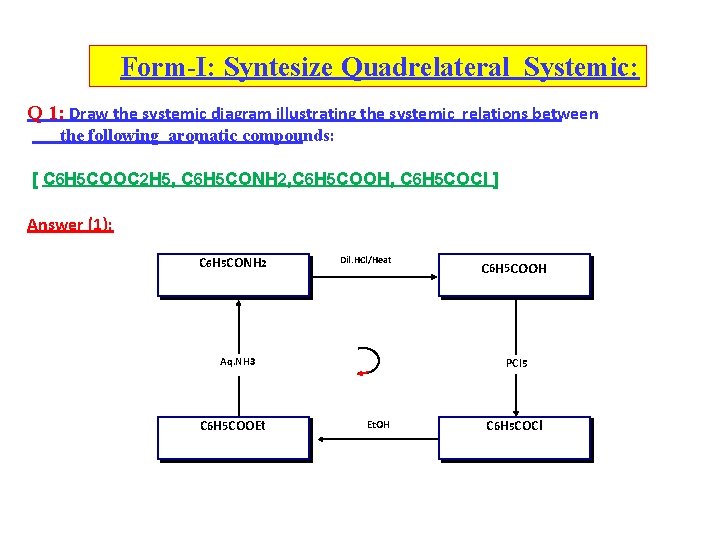
Form-I: Syntesize Quadrelateral Systemic: Q 1: Draw the systemic diagram illustrating the systemic relations between the following aromatic compounds: [ C 6 H 5 COOC 2 H 5, C 6 H 5 CONH 2, C 6 H 5 COOH, C 6 H 5 COCl ] Answer (1): C 6 H 5 CONH 2 Dil. HCl/Heat Aq. NH 3 C 6 H 5 COOEt C 6 H 5 COOH PCl 5 Et. OH C 6 H 5 COCl

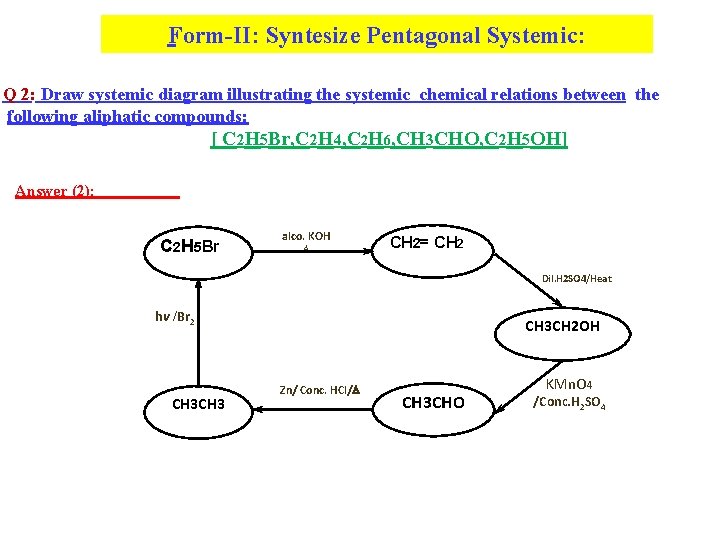
Form-II: Syntesize Pentagonal Systemic: Q 2: Draw systemic diagram illustrating the systemic chemical relations between the following aliphatic compounds: [ C 2 H 5 Br, C 2 H 4, C 2 H 6, CH 3 CHO, C 2 H 5 OH] Answer (2): C 2 H 5 Br alco. KOH CH 2= CH 2 Dil. H 2 SO 4/Heat hv /Br 2 CH 3 CH 2 OH Zn/ Conc. HCl/ CH 3 CHO KMn. O 4 /Conc. H 2 SO 4
![Type6 Systemic Analysis Questions SSAn Q s Requires the Student to Analyze the given Type-6: Systemic Analysis Questions [SSAn. Q, s] Requires the Student to Analyze the given](https://slidetodoc.com/presentation_image/bb63d05768f3b3049cafbf15e6eeaeca/image-27.jpg)
Type-6: Systemic Analysis Questions [SSAn. Q, s] Requires the Student to Analyze the given systemic diagram to its concepts or formula, numbers, or events with their relations.

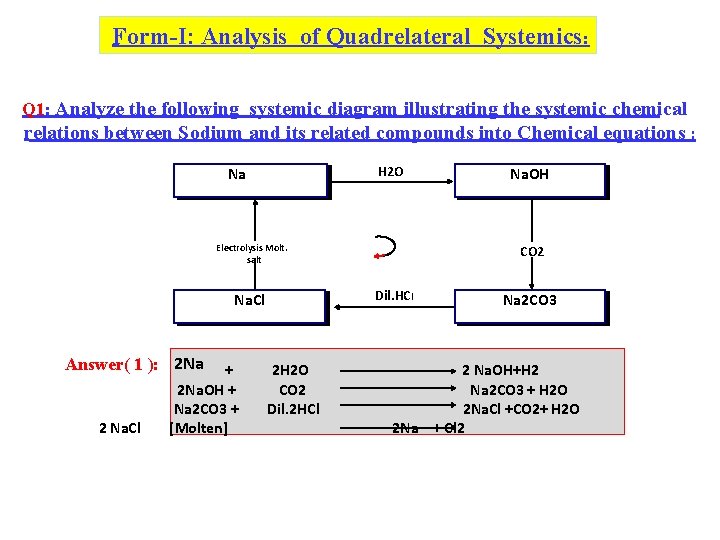
Form-I: Analysis of Quadrelateral Systemics: Q 1: Analyze the following systemic diagram illustrating the systemic chemical relations between Sodium and its related compounds into Chemical equations : H 2 O Na Electrolysis Molt. salt Answer( 1 ): 2 Na 2 Na. Cl + 2 Na. OH + Na 2 CO 3 + [Molten] CO 2 Dil. HCl Na. Cl 2 H 2 O CO 2 Dil. 2 HCl Na. OH Na 2 CO 3 2 Na. OH+H 2 Na 2 CO 3 + H 2 O 2 Na. Cl +CO 2+ H 2 O 2 Na + Cl 2

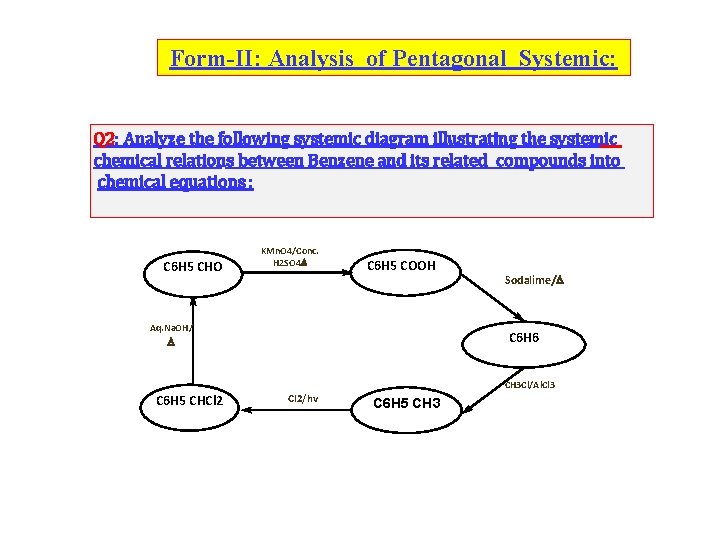
Form-II: Analysis of Pentagonal Systemic: Q 2: Analyze the following systemic diagram illustrating the systemic chemical relations between Benzene and its related compounds into chemical equations : C 6 H 5 CHO KMn. O 4/Conc. H 2 SO 4 C 6 H 5 COOH Aq. Na. OH/ Sodalime/ C 6 H 6 CH 3 Cl/Al. Cl 3 C 6 H 5 CHCl 2/hv C 6 H 5 CH 3

References: 1 -Fahmy, A. F. M. , Lagowski, J. J; Systemic Reform in Chemical Education , An International Perspective, J. Chem. Edu. , 2003, 80 (9), 1078. 2 -Fahmy, A. F. M. , Lagowski, J. J. , Using SATL Techniques to Assess Student Achievement, [18 th ICCE, Istanbul Turkey, 3 -8, August 2004]. 3 -ahmy, A. F. M. , Lagowski, J. J. , ” Systemic multiple choice questions (SMCQs) in Chemistry “[19 th ICCE, Seoul, South Korea, 12 -17 August 2006]. 4 - Systemic Multiple Choice Questions in Chemistry. A. F. M. Fahmy, J. J. Lagowski Chemical Education International Vol. 3 (2006/2008) http: //www. iupac. org/publications/cei 5 - Systemic assessment as a new tool for assessing students learning In Chemistry using SATL methods: Systemic true false [STFQs] and systemic sequencing [SSQs] question types. A. F. M. Fahmy and J. J. Lagowski AJCE. 2, (2)66 -78(2012).

6 -Exploring novel tools for assessing high School students' meaningful understanding of organic reactions, Vachliotis, T, Salta, K. , Vasiliou, P. , Tzougraki, C J. Chem. Educ. 88 [3] , 337 -345. (2011 7 -Colleen M. and Bull, J. ; Workshop on designing objective test questions. CAA center (http: //caacenter. ac. uk/ university of luton, luton UK. . 8 -Writing Multiple Choice Items which Require Comprehensio by Russell A. Dewey, Ph. D http: //www. psychwww. com/selfquiz/aboutq. htm 9 -Mc. Millan, J. H. (2001). Classroom assessment Principles and practice for effective instruction. Boston: Allyn and Bacon. . 10 - http: //web. utk. edu/~mccay/apdm/match_b. htm

 Systemic assessment examples
Systemic assessment examples Cosmic superclass
Cosmic superclass Ap diameter of chest
Ap diameter of chest Hr planning process
Hr planning process What is tactile fremitus
What is tactile fremitus How to assess alert and oriented
How to assess alert and oriented Tcrm steps
Tcrm steps Abbott nutrition focused physical assessment
Abbott nutrition focused physical assessment How to assess alert and oriented
How to assess alert and oriented Organised retailing
Organised retailing Tactile fermitus
Tactile fermitus Aatsecureassess
Aatsecureassess Sqa solar open assess
Sqa solar open assess Projective test psychology definition
Projective test psychology definition Blocked random assignment
Blocked random assignment City & guilds evolve
City & guilds evolve Evolve secure assess
Evolve secure assess Cranial nerve to smile
Cranial nerve to smile Eşleştirme sorusu
Eşleştirme sorusu Plan do review template
Plan do review template Assess plan act
Assess plan act Tectonic hazard profile
Tectonic hazard profile Acfi assessment pack
Acfi assessment pack Hypoglossal cranial nerve test
Hypoglossal cranial nerve test Why assess
Why assess Fedcb
Fedcb Techniques to assess country risk
Techniques to assess country risk Ask assess advise
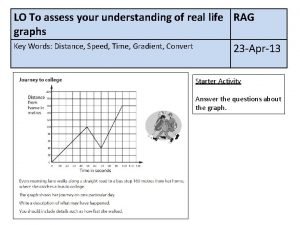
Ask assess advise Distance time graph activity
Distance time graph activity To assess achievement at the end of instruction is
To assess achievement at the end of instruction is Assess plan do review model
Assess plan do review model Humanistic psychologists may assess personality by
Humanistic psychologists may assess personality by Kontos systemic miticide
Kontos systemic miticide