Synthesis of 5 thioDglucose acetone pyridine water pyridine






- Slides: 14

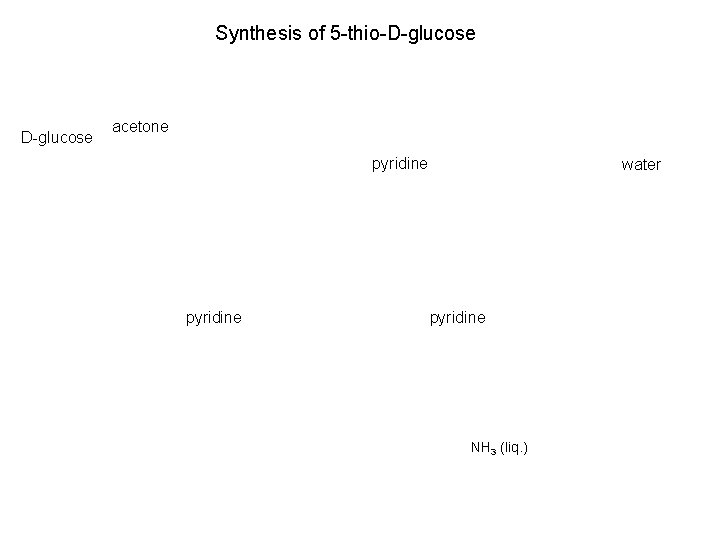
Synthesis of 5 -thio-D-glucose acetone pyridine water pyridine NH 3 (liq. )

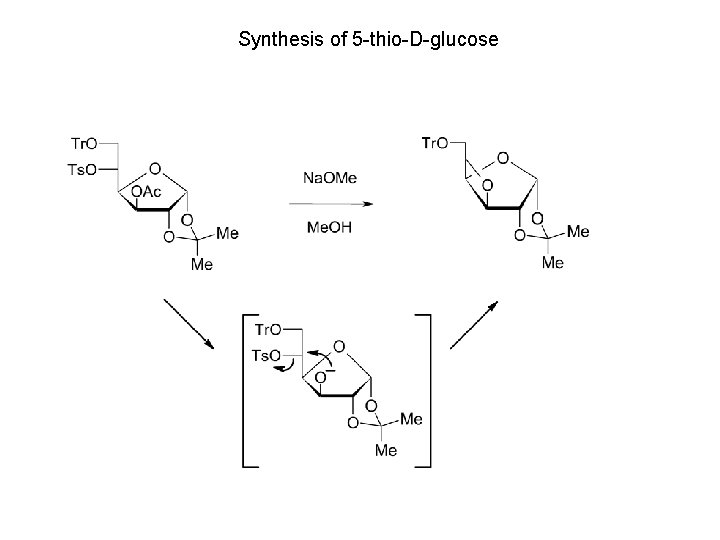
Synthesis of 5 -thio-D-glucose

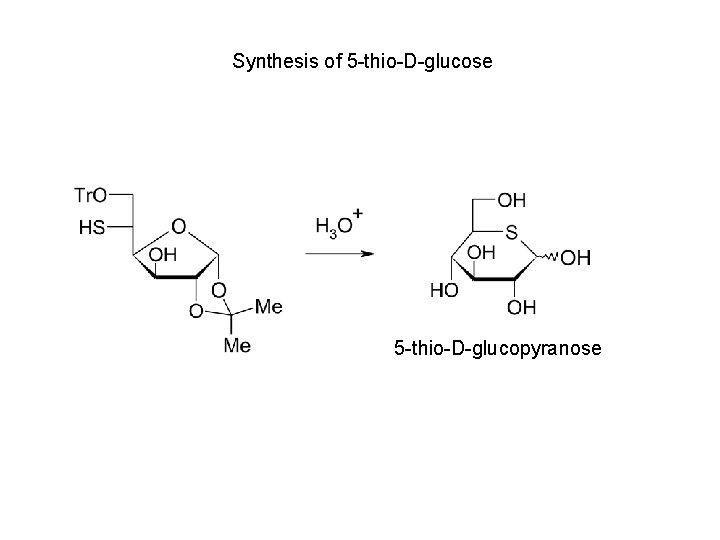
Synthesis of 5 -thio-D-glucose 5 -thio-D-glucopyranose

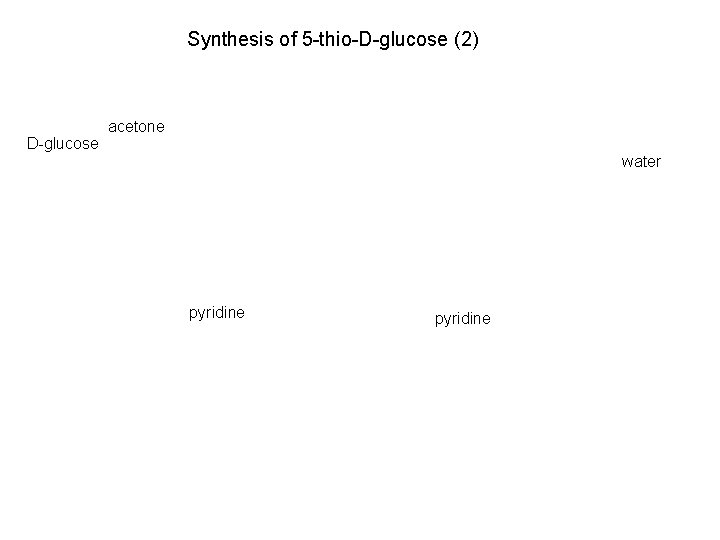
Synthesis of 5 -thio-D-glucose (2) D-glucose acetone water pyridine

Synthesis of 5 -thio-D-glucose (2)

Synthesis of 5 -thio-D-glucose (2) 5 -thio-D-glucopyranose

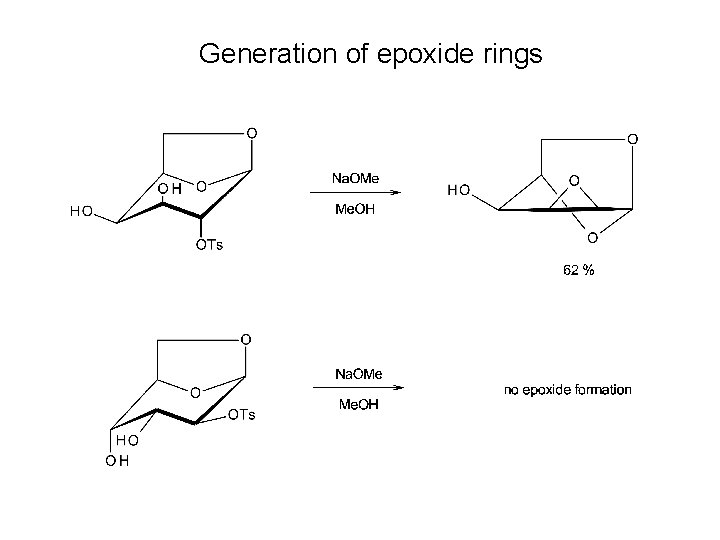
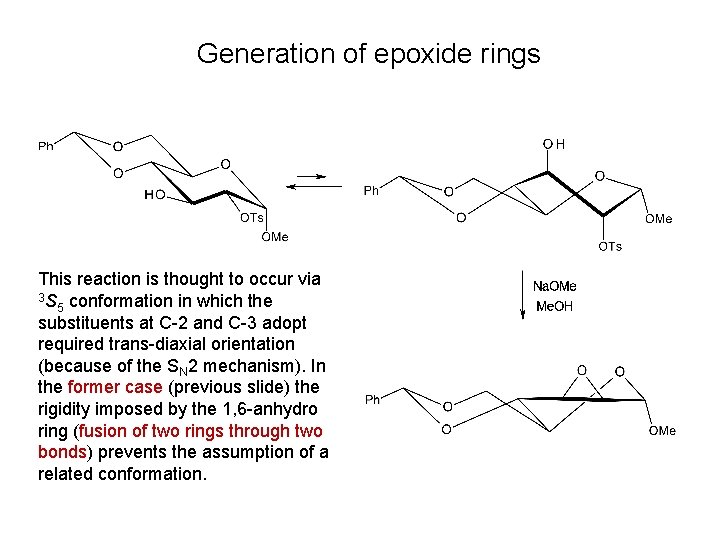
Generation of epoxide rings

Generation of epoxide rings This reaction is thought to occur via 3 S conformation in which the 5 substituents at C-2 and C-3 adopt required trans-diaxial orientation (because of the SN 2 mechanism). In the former case (previous slide) the rigidity imposed by the 1, 6 -anhydro ring (fusion of two rings through two bonds) prevents the assumption of a related conformation.

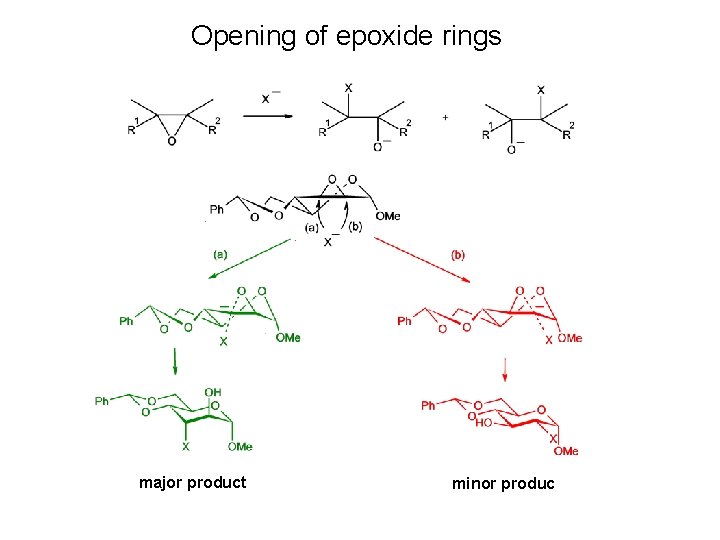
Opening of epoxide rings

Opening of epoxide rings

Opening of epoxide rings

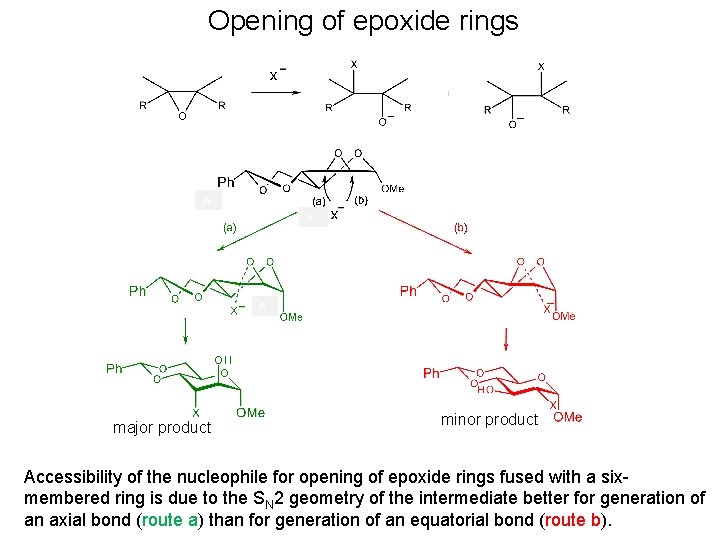
Opening of epoxide rings major product minor produc

Opening of epoxide rings • When the epoxide rings are fused in conformationally restricted derivatives such as 1, 6 -anhydropyranoses or transfused 4, 6 -O-benzylidenehexopyranosides, pyranoid rings adopt half-chair conformations, and the diastereoisomers having diaxial arrangement at the epoxide ring opened positions represents more than 90 % of the products of the nucleophilic opening of the epoxide ring. • This is in accordance with the Fürst-Plattner rule, formulated from studies of the nucleophilic opening of steroidal epoxides, which can be explained by consideration of the transition states in the ring opening reactions.

Opening of epoxide rings A A A major product minor product Accessibility of the nucleophile for opening of epoxide rings fused with a sixmembered ring is due to the SN 2 geometry of the intermediate better for generation of an axial bond (route a) than for generation of an equatorial bond (route b).
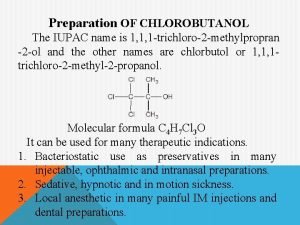
 To prepare and submit chlorobutanol from acetone
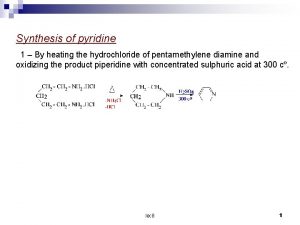
To prepare and submit chlorobutanol from acetone Pyridine synthesis from 1 5 dicarbonyl
Pyridine synthesis from 1 5 dicarbonyl Preparation of pyridine from acetylene
Preparation of pyridine from acetylene Water and water and water water
Water and water and water water Acetone breath not diabetes
Acetone breath not diabetes Acetone fixative
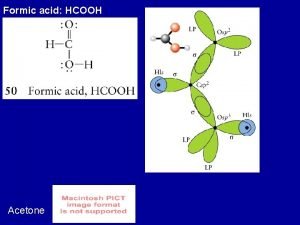
Acetone fixative Hcooh hybridization of c
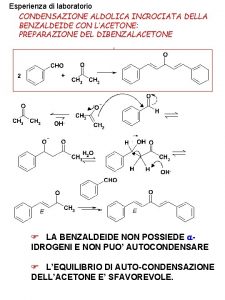
Hcooh hybridization of c Aldolica incrociata
Aldolica incrociata Acetone 密度
Acetone 密度 Styrofoam and acetone chemical or physical change
Styrofoam and acetone chemical or physical change (ch3)2nh lewis structure
(ch3)2nh lewis structure Campbell biology
Campbell biology Acetone hybridization
Acetone hybridization Ir spectroscopy
Ir spectroscopy Acetone in perfume
Acetone in perfume