Stoichiometry Adjusting To Reality Adjusting To Reality This











- Slides: 13

Stoichiometry Adjusting To Reality

Adjusting To Reality This is not the entire story. In reality, you never have the exact amounts of both reactants you need. At the end of the reaction, one reactant has been completely consumed and there is some “left over” of the other reactant. Let’s take a second look at the reaction between Pb. S and O 2.

2 Pb. S + 3 O 2 2 Pb. O + 2 SO 2 Since oxygen is not costly (free in our atmosphere), it’s usually the reactant there is plenty of and only a certain amount of lead II sulfide would have been purchased for this reaction. What would our BCA table look like if we had 0. 40 moles of lead (II) sulfide reacting with an abundance (excess) of oxygen?

Adjusting To Reality Equation: 2 Pb. S + 3 O 2 2 Pb. O + 2 SO 2 Before: . 40 mol xs mol 0 mol Change -. 40 mol - xs mol +. 40 mol __________________ After 0 mol xs mol. 40 mol Excess is written (xs) and indicates there is some reactant remaining. Here, Pb. S is completely consumed and some O 2 remains after the reaction is complete.

Further Reality What if only a certain amount of each reactant were available? 25. 50 g of oxygen reacts with 114. 85 g lead (II) sulfide producing lead (II) oxide and sulfur dioxide. What mass of lead (II) oxide would be produced? We cannot directly measure moles, so the reactant amounts are given in grams. In order to use our BCA table (for mole ratios) we need the amounts in moles. Using molar mass, we convert the mass of the reactants to moles of reactants.

Mass To Moles, “Molar Mass” 25. 50 g O 2 x 1 mol O 2_ =. 80 mol O 2 32. 0 g O 2 114. 85 g Pb. S x _1 mol Pb. S_ =. 48 mol Pb. S 239. 27 g Pb. S

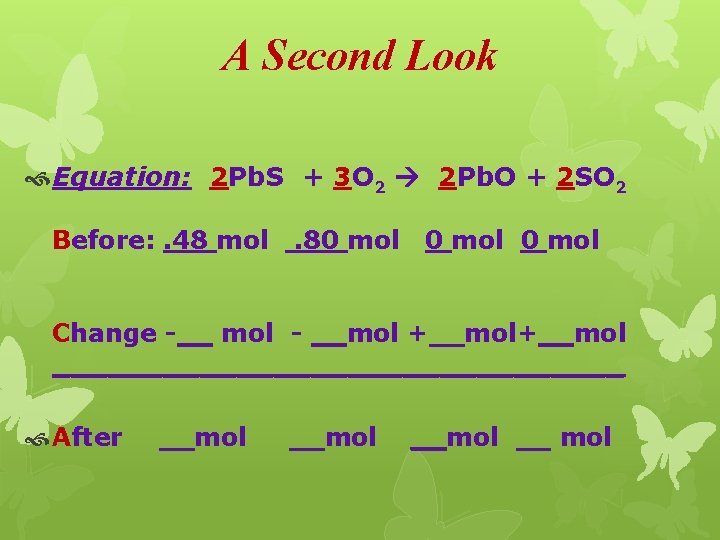
A Second Look Equation: 2 Pb. S + 3 O 2 2 Pb. O + 2 SO 2 Before: . 48 mol . 80 mol Change -__ mol - __mol +__mol ________________ After __mol __ mol

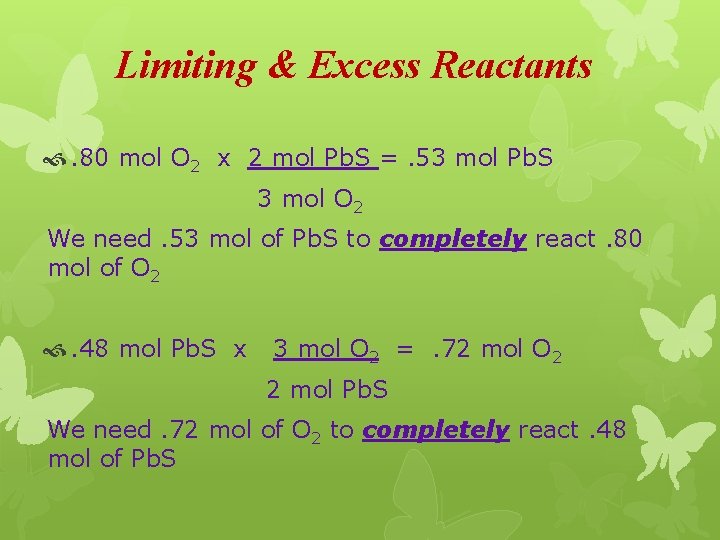
Limiting & Excess Reactants Our first task is to find out which reactant will be completely consumed (limiting reactant) and which reactant will have some remaining after the reaction is complete (reactant in excess). We will use mole ratios from the BCA table for this task.

Limiting & Excess Reactants . 80 mol O 2 x 2 mol Pb. S =. 53 mol Pb. S 3 mol O 2 We need. 53 mol of Pb. S to completely react. 80 mol of O 2 . 48 mol Pb. S x 3 mol O 2 =. 72 mol O 2 2 mol Pb. S We need. 72 mol of O 2 to completely react. 48 mol of Pb. S

Evaluating Our Answers We need 0. 53 mol Pb. S to completely burn 0. 80 mol O 2. We only have 0. 48 mol Pb. S. Not all of the O 2 will be “consumed”. The reaction will stop when the Pb. S has run out. This tells us the Pb. S will limit the reaction (limiting reactant) and some oxygen will remain after the reaction is complete (reactant in excess).

Amount of Reactant Remaining We need 0. 72 mol O 2 to completely react with 0. 48 mol Pb. S. We have 0. 80 mol O 2. 0. 08 mol O 2 will remain after all of the Pb. S has been consumed.

Importance of Limiting Reactant The Pb. S limits the reaction. Pb. S “runs out” before all the O 2 is consumed. Pb. S is the reactant that determines how much product will be produced.

Putting It All Together! Equation: 2 Pb. S Before: . 48 mol + 3 O 2 . 80 mol 2 Pb. O + 2 SO 2 0 mol Change -. 48 mol -. 72 mol +. 48 mol __________________ After: 0 mol. 08 mol. 48 mol
 Limiting reactant def
Limiting reactant def Why is evaluating and adjusting a spending plan important
Why is evaluating and adjusting a spending plan important Adjusting entries affect the cash account
Adjusting entries affect the cash account Contoh journal entries
Contoh journal entries Adjusting entries
Adjusting entries Accrued revenue adjusting entry
Accrued revenue adjusting entry Adjusting entry accrued expense
Adjusting entry accrued expense Adjusting entries for merchandise inventory
Adjusting entries for merchandise inventory Fungsi idle mixture adjusting screw
Fungsi idle mixture adjusting screw Adjusting and closing entries
Adjusting and closing entries Adjusting the accounts chapter 3
Adjusting the accounts chapter 3 The updating of accounts is called the adjusting process.
The updating of accounts is called the adjusting process. Which types of adjusting entries are natural opposites?
Which types of adjusting entries are natural opposites? Accrued expenses are
Accrued expenses are