Signal Detection vigilance data Content What is Signal








- Slides: 13

Signal Detection – vigilance data

Content – What is Signal detection / management / definitions – The Process – The HPRA Process 04/01/2022 2


Article 65 a Analysis of Vigilance Data • The Commission shall, in collaboration with the Member States, put in place systems and processes to proactively monitor the data available in the database referred to in Article 66 a, in order to identify trends, patterns or signals in the data that may identify new risks or safety concerns. • When a previously unknown risk is identified or the frequency of an anticipated risk significantly and adversely changes the risk-benefit determination, the competent authority or, where appropriate, the coordinating competent authority shall inform the manufacturer, or where applicable the authorised representative, who shall take the necessary corrective actions. date Pied de page 4

Safety Signal • “A report or reports of an event with an unknown causal relationship to treatment that is recognized as worthy of further exploration and continued surveillance. ” Council for International Organizations of Medical Sciences (CIOMS) • “Recognition” is often the results of Analytical or automatic signal detection methods that look for unexpected patterns in data sources such as: – Spontaneously reported data – Observational healthcare data – Insurance claims data date Pied de page 5

The Signal Management Process • The signal management process can be defined as the set of activities performed to determine whether, based on an examination of individual case safety reports, aggregated data from active surveillance systems or studies, literature information or other data sources, there are new risks associated with a medical device or whether known risks have changed. date Pied de page 6

The Signal Management Process • The signal management process can include all steps from – signal detection; – signal validation; – signal analysis and prioritisation; – signal assessment; – recommendation for action; – exchange of information. date Pied de page 7

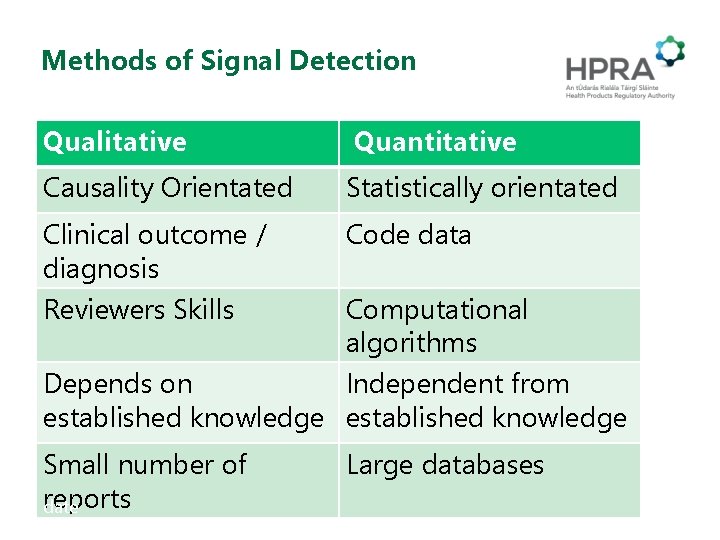
Methods of Signal Detection Qualitative Quantitative Causality Orientated Statistically orientated Clinical outcome / diagnosis Reviewers Skills Code data Small number of reports date Large databases Computational algorithms Depends on Independent from established knowledge 8

Methods of Validation • Confirm product sales / incident rates with the manufacturer • Central EU Database • International Databases • The literature • Vigilance enquiry form date 9

Recommendation for Action Close No Risk No Action Needed date New Risk IFU Update Pied de page Immediate Action Product Recall Periodic review PMS Studies 10

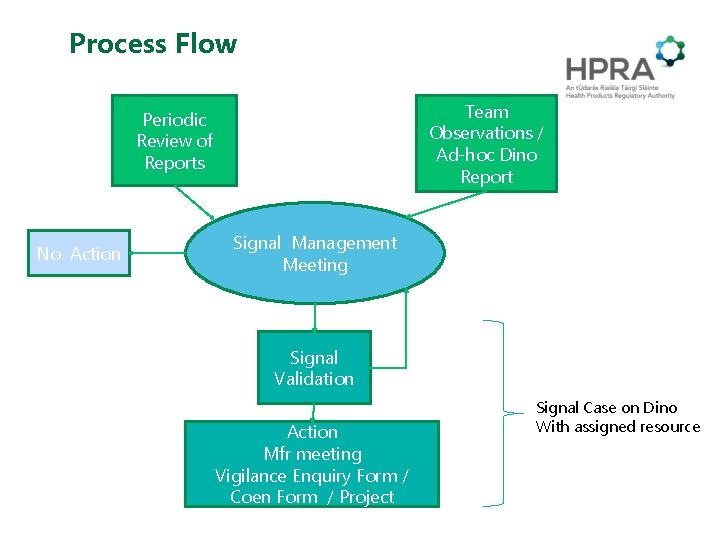
Process Flow Team Observations / Ad-hoc Dino Report Periodic Review of Reports No Action Signal Management Meeting Signal Validation date Action Mfr meeting Vigilance Enquiry Form / Coen Form / Project Pied de page Signal Case on Dino With assigned resource 11

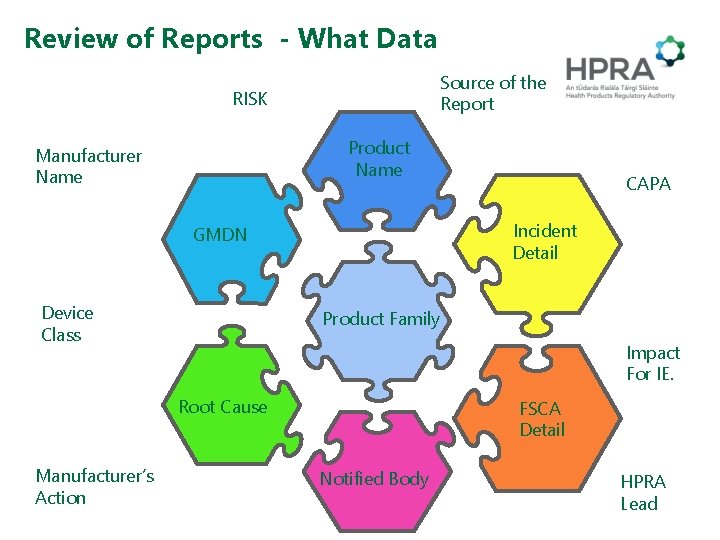
Review of Reports - What Data Source of the Report RISK Product Name Manufacturer Name Incident Detail GMDN Device Class Product Family Impact For IE. Root Cause Manufacturer’s Action CAPA FSCA Detail Notified Body HPRA Lead

Causality Thank you Pied de page date 13