Short Announcements 1 st Quiz today Homework 2








- Slides: 25

Short Announcements 1 st Quiz today Homework #2 on web. Due next Monday. Chpt 2 Reading Due next Wednesday (NOT Monday) Today’s Lecture: Protein Folding

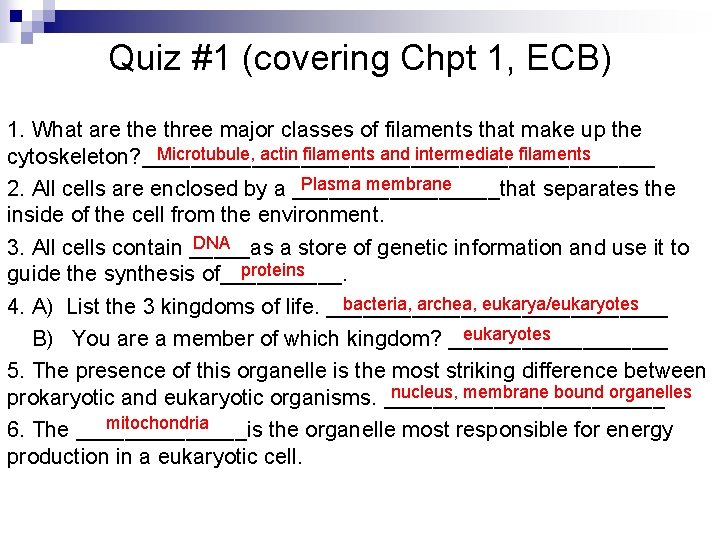
Quiz #1 (covering Chpt 1, ECB) 1. What are three major classes of filaments that make up the Microtubule, actin filaments and intermediate filaments cytoskeleton? _____________________ Plasma membrane 2. All cells are enclosed by a _________that separates the inside of the cell from the environment. DNA 3. All cells contain _____as a store of genetic information and use it to proteins guide the synthesis of_____. bacteria, archea, eukarya/eukaryotes 4. A) List the 3 kingdoms of life. ______________ eukaryotes B) You are a member of which kingdom? _________ 5. The presence of this organelle is the most striking difference between nucleus, membrane bound organelles prokaryotic and eukaryotic organisms. ____________ mitochondria 6. The _______is the organelle most responsible for energy production in a eukaryotic cell.

The Protein (Free) Energy Landscape Largely from Martin Gruebele, Chemistry, Physics UIUC

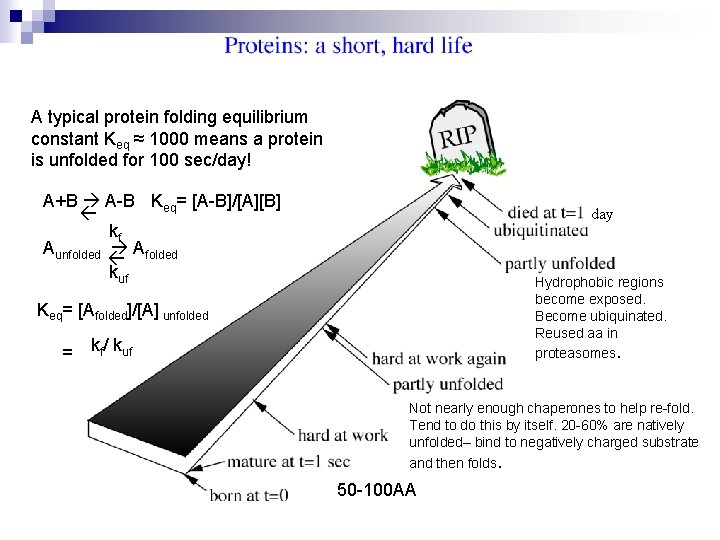
A typical protein folding equilibrium constant Keq ≈ 1000 means a protein is unfolded for 100 sec/day! A+B A-B Keq= [A-B]/[A][B] kf Afolded kuf Aunfolded day Hydrophobic regions become exposed. Become ubiquinated. Reused aa in proteasomes. Keq= [Afolded]/[A] unfolded = kf/ kuf Not nearly enough chaperones to help re-fold. Tend to do this by itself. 20 -60% are natively unfolded– bind to negatively charged substrate and then folds. 50 -100 AA

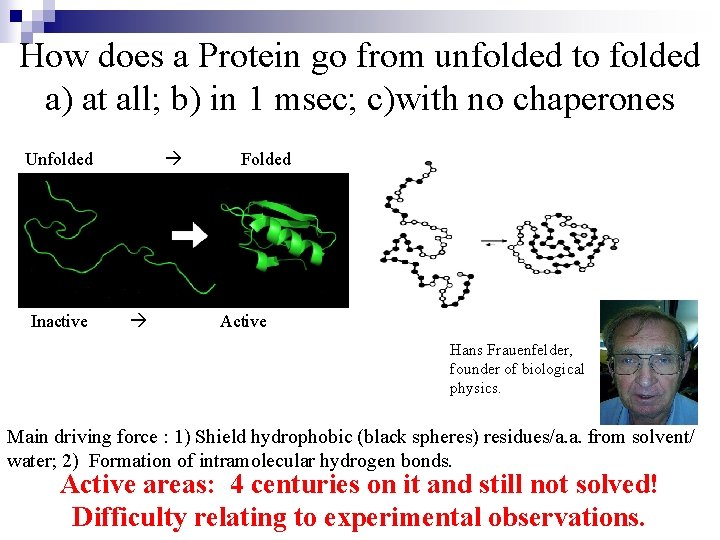
How does a Protein go from unfolded to folded a) at all; b) in 1 msec; c)with no chaperones Unfolded Inactive Folded Active Hans Frauenfelder, founder of biological physics. Main driving force : 1) Shield hydrophobic (black spheres) residues/a. a. from solvent/ water; 2) Formation of intramolecular hydrogen bonds. Active areas: 4 centuries on it and still not solved! Difficulty relating to experimental observations.

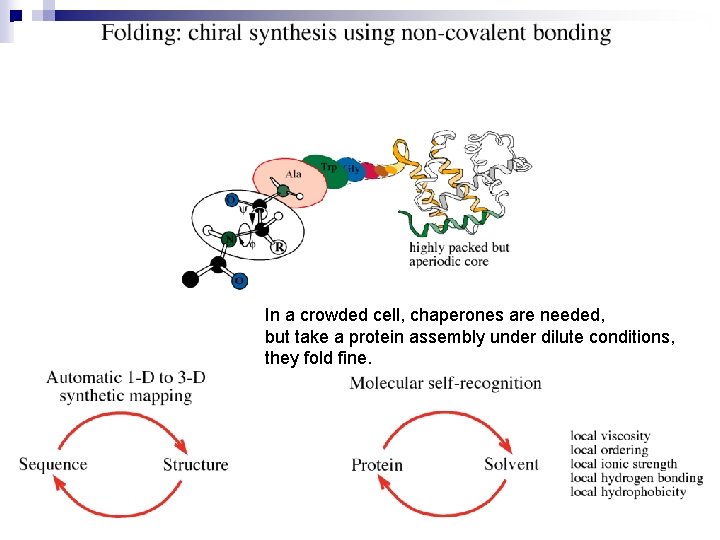
In a crowded cell, chaperones are needed, but take a protein assembly under dilute conditions, they fold fine.

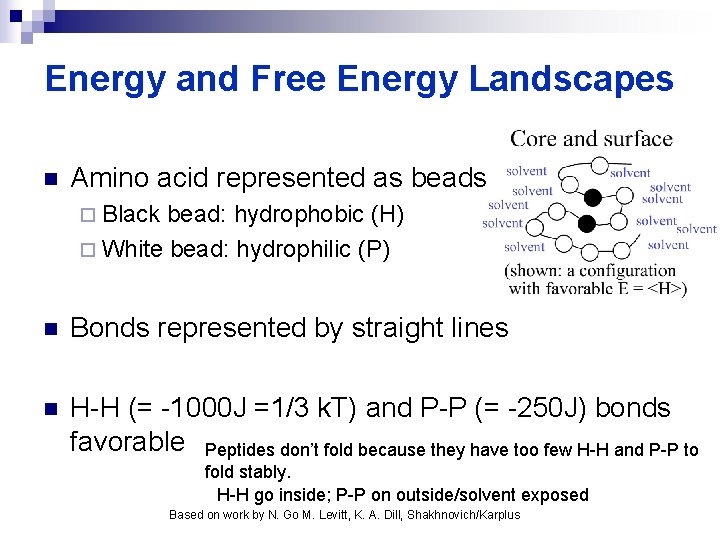
Energy and Free Energy Landscapes n Amino acid represented as beads ¨ Black bead: hydrophobic (H) ¨ White bead: hydrophilic (P) n Bonds represented by straight lines n H-H (= -1000 J =1/3 k. T) and P-P (= -250 J) bonds favorable Peptides don’t fold because they have too few H-H and P-P to fold stably. H-H go inside; P-P on outside/solvent exposed Based on work by N. Go M. Levitt, K. A. Dill, Shakhnovich/Karplus

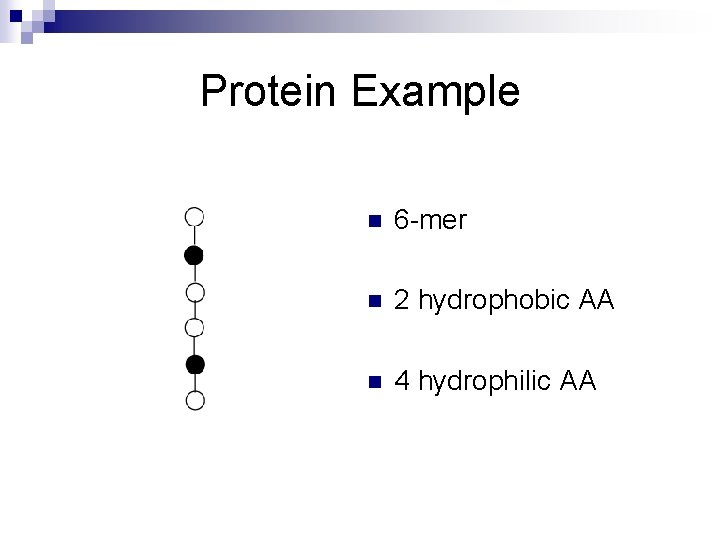
Protein Example n 6 -mer n 2 hydrophobic AA n 4 hydrophilic AA

Chirality in Amino acids Although most amino acids can exist in both left and right handed forms, Life on Earth is made of left handed amino acids, almost exlusively. Why? Not really known. Meteorites have lefthanded aa. http: //en. wikipedia. org/wiki/File: Chi rality_with_hands. jpg n n To avoid issues with chirality, all molecules are made so that the first two amino acids go upwards. Also, the first kink always goes to the right.

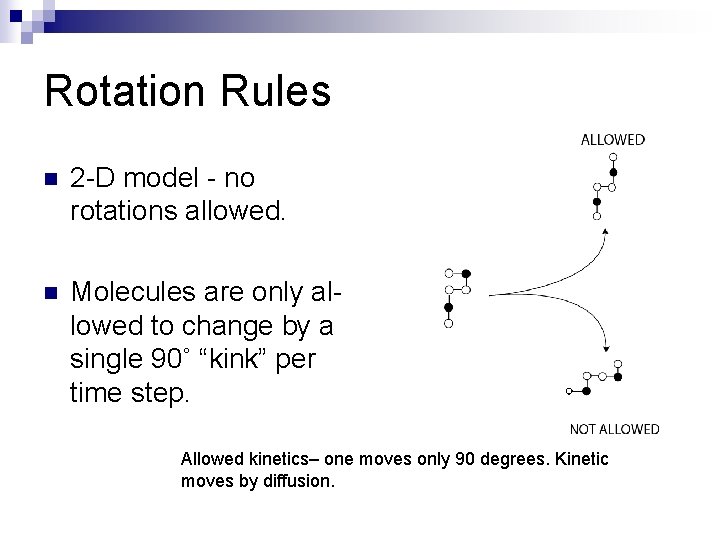
Rotation Rules n 2 -D model - no rotations allowed. n Molecules are only allowed to change by a single 90˚ “kink” per time step. Allowed kinetics– one moves only 90 degrees. Kinetic moves by diffusion.

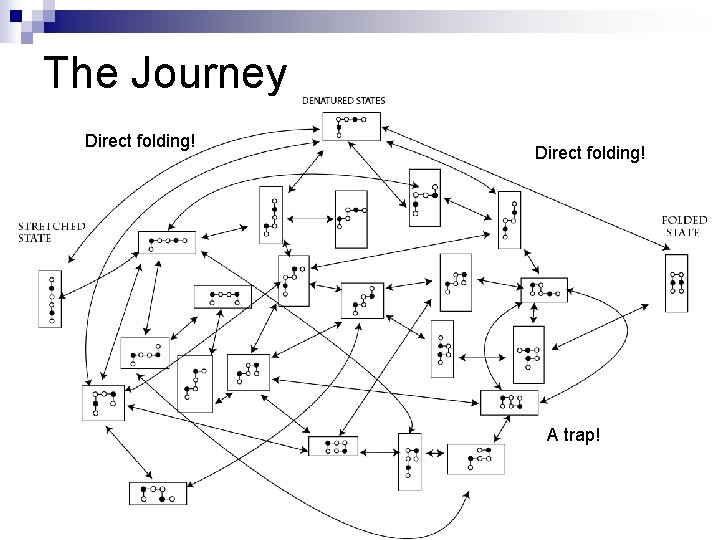
The Journey Direct folding! A trap!

Entropy

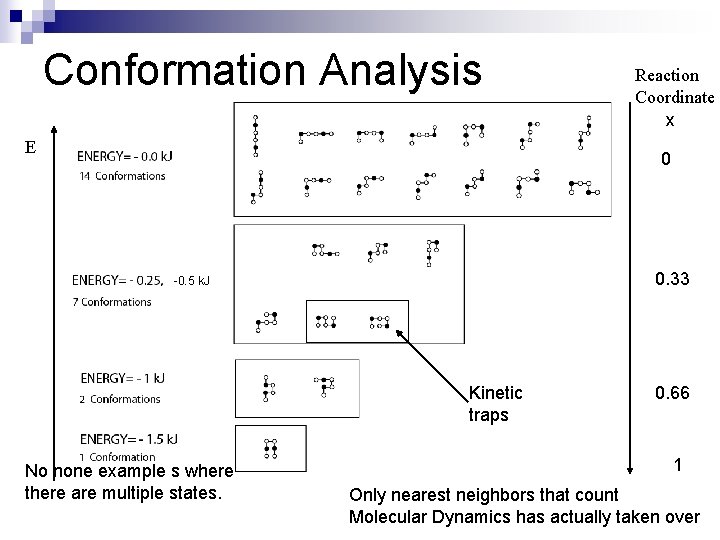
Conformation Analysis E Reaction Coordinate x 0 0. 33 -0. 5 k. J Kinetic traps No none example s where there are multiple states. 0. 66 1 Only nearest neighbors that count Molecular Dynamics has actually taken over

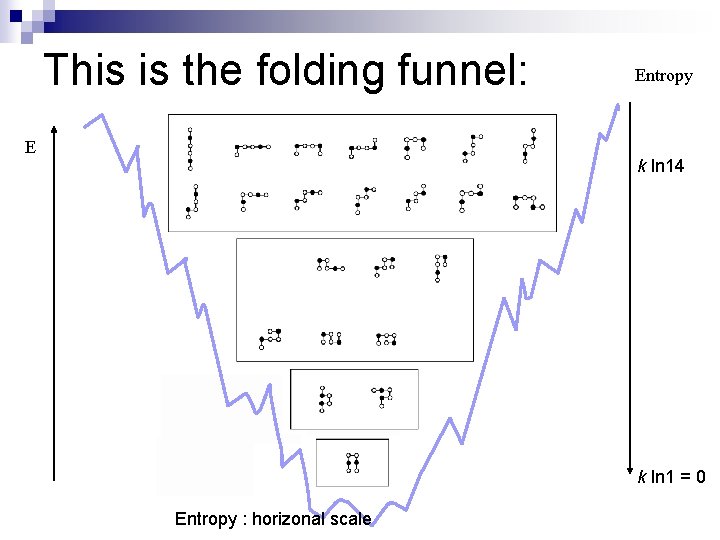
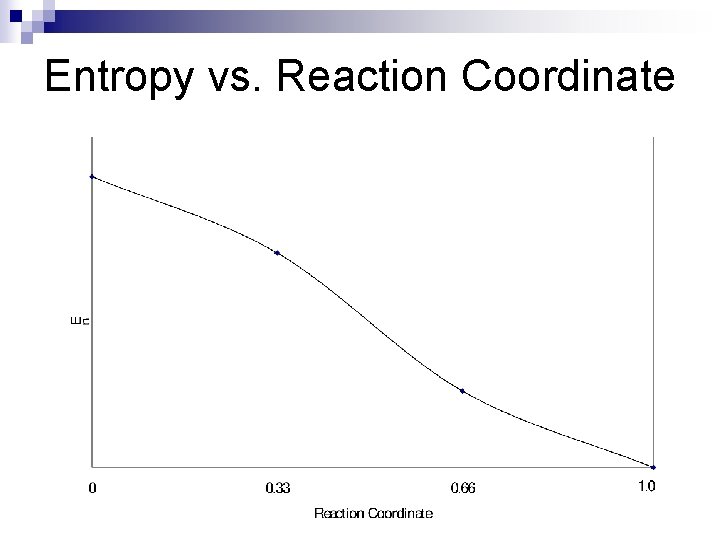
This is the folding funnel: E Entropy k ln 14 k ln 1 = 0 Entropy : horizonal scale

Entropy vs. Energy correlated monotonic function Ln 14 Ln 1

Entropy vs. Reaction Coordinate

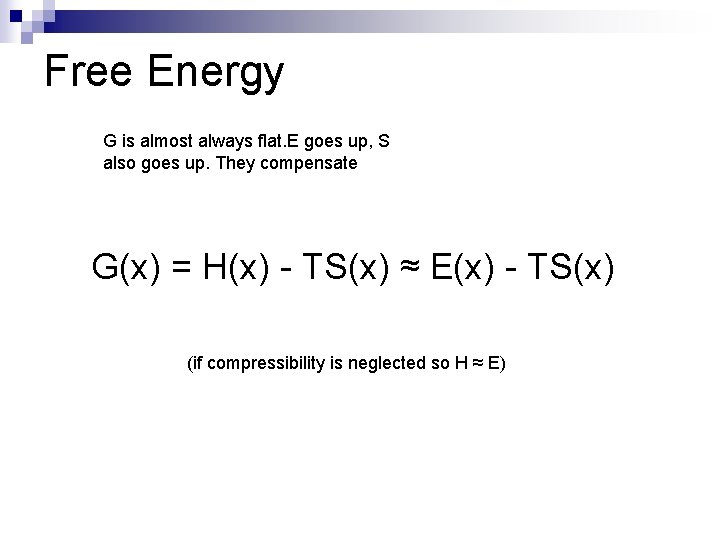
Free Energy G is almost always flat. E goes up, S also goes up. They compensate G(x) = H(x) - TS(x) ≈ E(x) - TS(x) (if compressibility is neglected so H ≈ E)

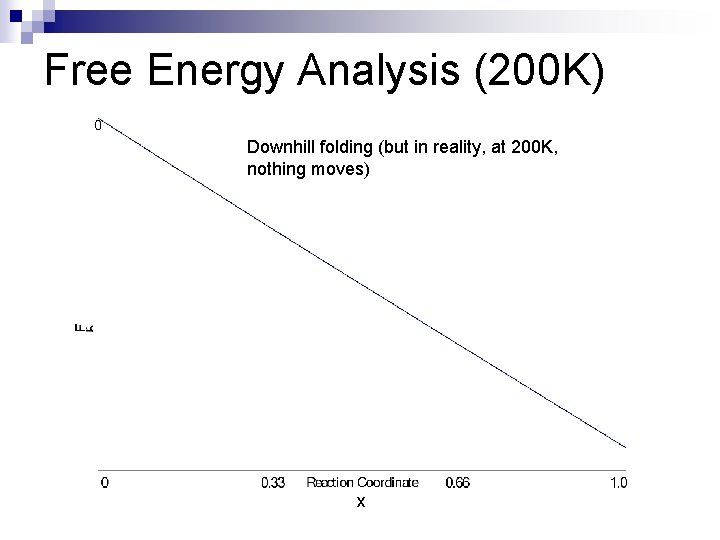
Free Energy Analysis (200 K) Downhill folding (but in reality, at 200 K, nothing moves) x

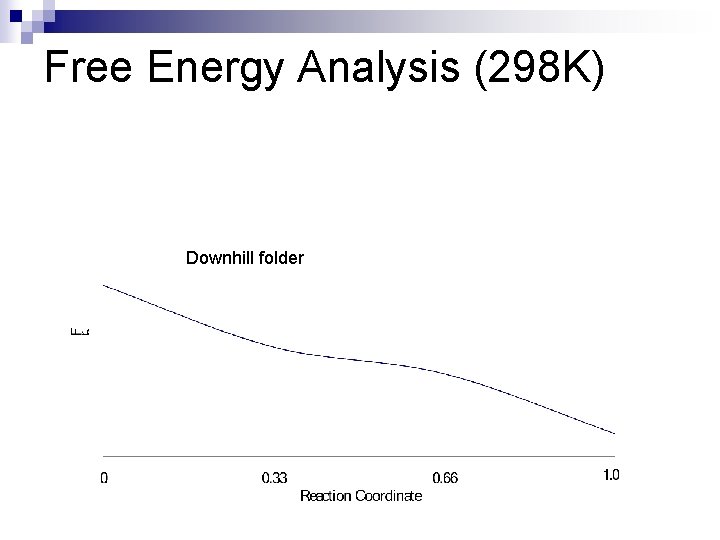
Free Energy Analysis (298 K) Downhill folder

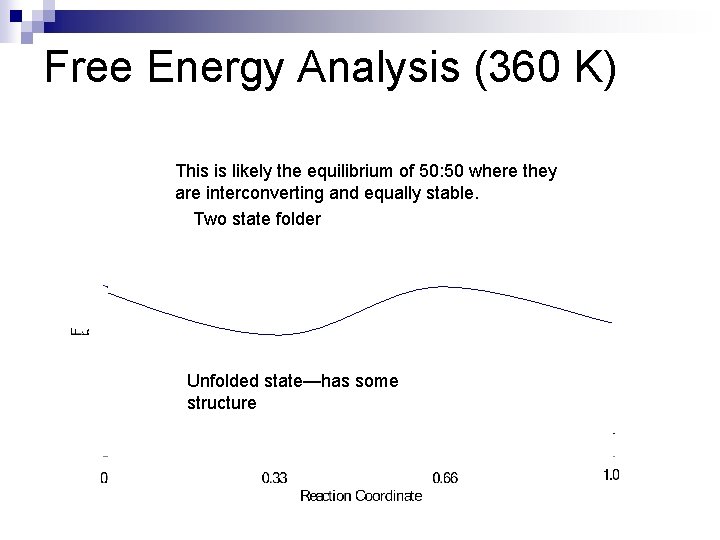
Free Energy Analysis (360 K) This is likely the equilibrium of 50: 50 where they are interconverting and equally stable. Two state folder Unfolded state—has some structure

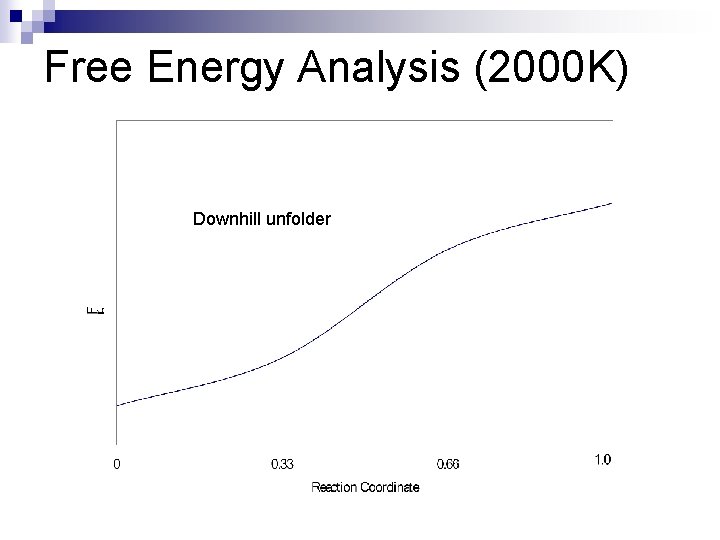
Free Energy Analysis (2000 K) Downhill unfolder

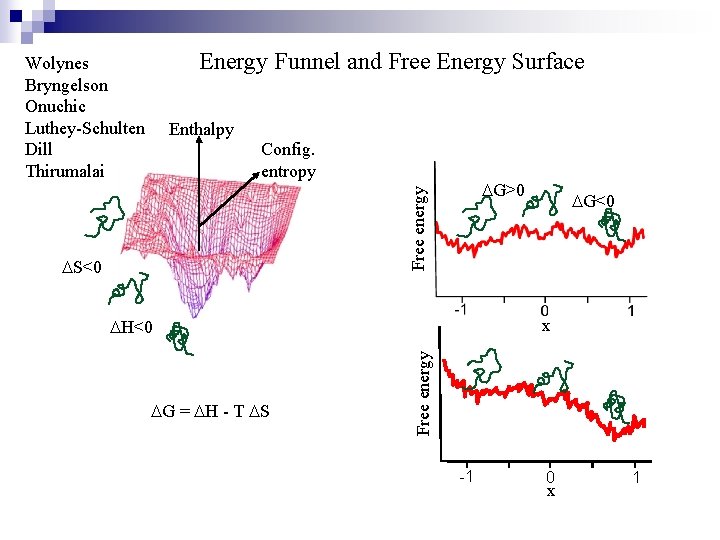
Energy Funnel and Free Energy Surface Wolynes Bryngelson Onuchic Luthey-Schulten Dill Thirumalai Enthalpy Config. entropy Free energy DG>0 DS<0 x Free energy DH<0 DG = DH - T DS DG<0 -1 0 x 1

Amyloid Fibers…involved in Alzheimers There is a lower energy state which is fibers Protein amyloid fibers are often found to —e. g. ameloid fibers– mutliple states! have a β-pleated sheet structure regardless of their sequence, leading some to believe that it is the molecule's misfolding that leads to aggregation. http: //www. informaworld. com/smpp/content~content=a 7 79685983~db=medi~order=page Enzymes act on the APP (Amyloid precursor protein) and cut it into fragments of protein, one of which is called beta-amyloid and its crucial in the formation of senile plaques in Alzheimer

Summary of Protein Folding Proteins can fold. Don’t need chaperones. ΔG is always about zero. Therefore can fold fast. Kinetics – fast cause not huge barriers

Class evaluation 1. What was the most interesting thing you learned in class today? 2. What are you confused about? 3. Related to today’s subject, what would you like to know more about? 4. Any helpful comments. Answer, and turn in at the end of class.
 Long and short
Long and short Pvu background
Pvu background R/announcements
R/announcements David ritthaler
David ritthaler What does montag hear in the seashell
What does montag hear in the seashell Kluver bucy syndrome
Kluver bucy syndrome General announcements
General announcements Jack prelutsky homework oh homework
Jack prelutsky homework oh homework Homework oh homework i hate you you stink
Homework oh homework i hate you you stink Jack prelutsky homework oh homework
Jack prelutsky homework oh homework Homework oh homework i hate you you stink
Homework oh homework i hate you you stink Alitteration definition
Alitteration definition Example of literal language
Example of literal language Homework last night
Homework last night Homework due today
Homework due today Black cat analogy
Black cat analogy Homework due today
Homework due today Homework due today
Homework due today Iron core
Iron core Astr
Astr Homework due today
Homework due today Today meeting or today's meeting
Today meeting or today's meeting Have a class today
Have a class today Meeting objective
Meeting objective Fingerprint galton details
Fingerprint galton details Today's lesson or today lesson
Today's lesson or today lesson