Section 2 Types of Chemical Reactions Synthesis Reactions




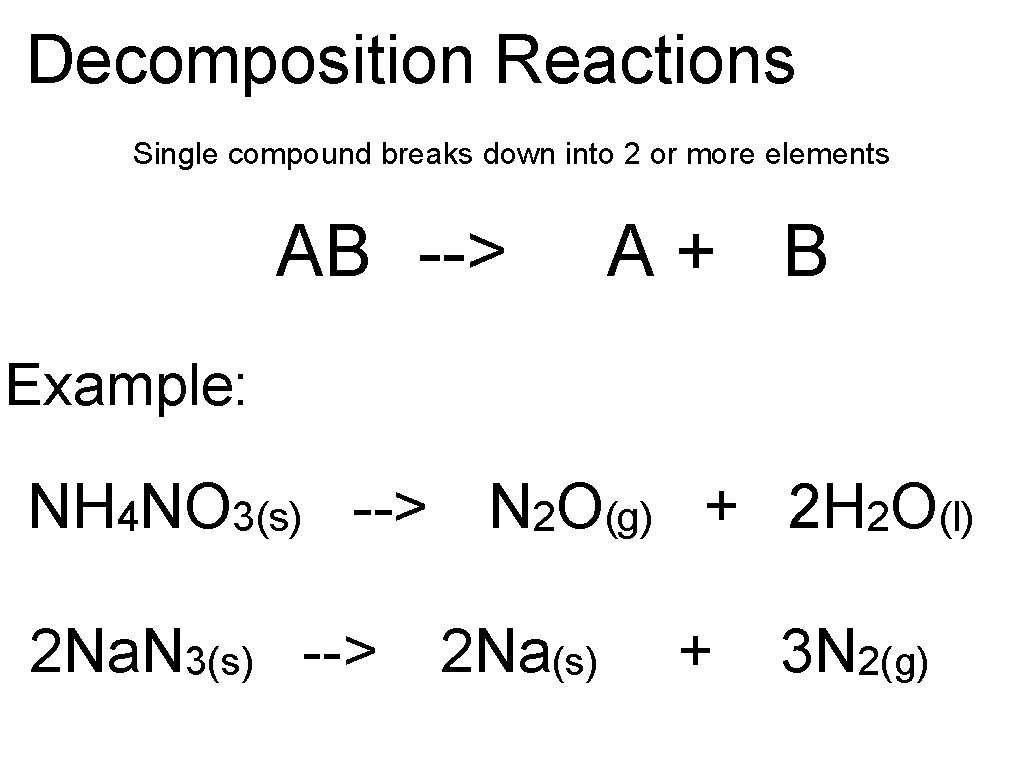



- Slides: 12

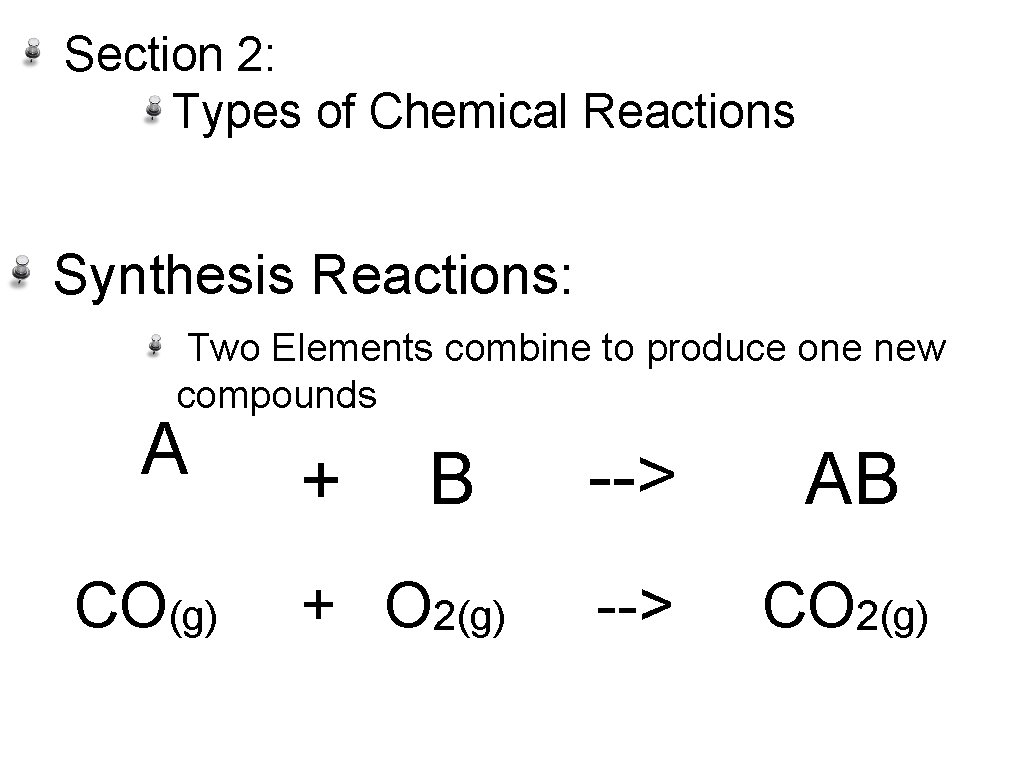
Section 2: Types of Chemical Reactions Synthesis Reactions: Two Elements combine to produce one new compounds A CO(g) + B + O 2(g) --> AB --> CO 2(g)

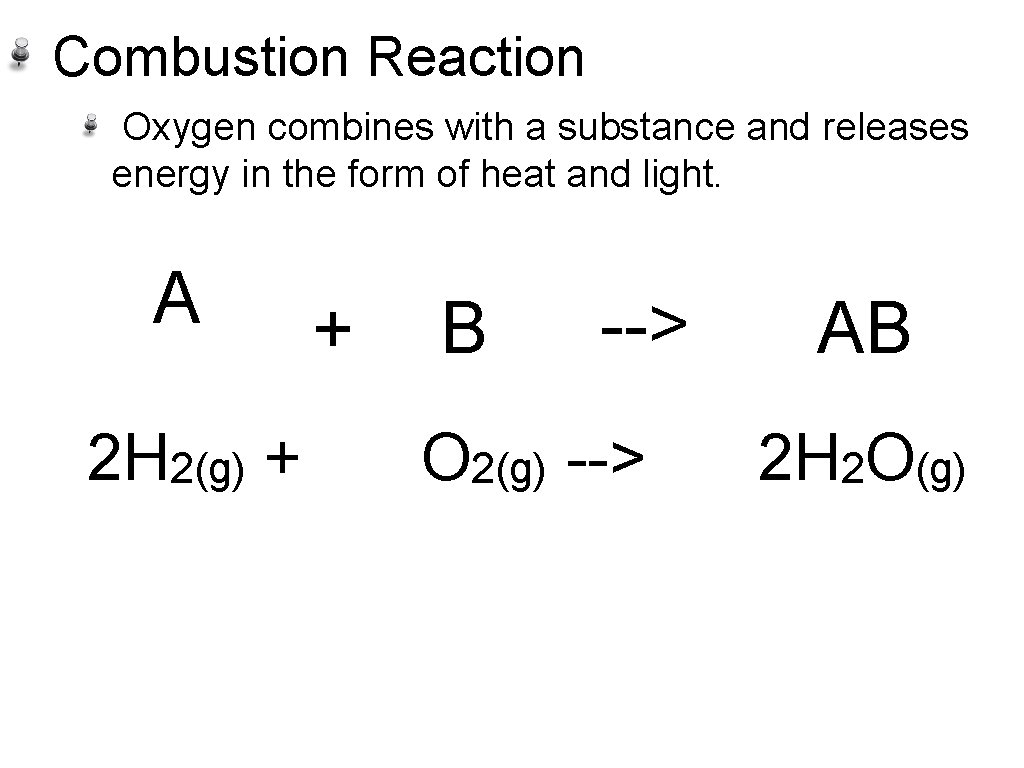
Combustion Reaction Oxygen combines with a substance and releases energy in the form of heat and light. A 2 H 2(g) + + B --> O 2(g) --> AB 2 H 2 O(g)

The solids aluminum and sulfur react to produce aluminum sulfide

Water and dinitrogen pentoxide gas react to produce aqueous hydrogen nitrate.

The gases nitrogen dioxide and oxygen react to produce dinitrogen pentoxide gas.

Ethane gas (C 2 H 6) burns in air, producing carbon dioxide gas and water vapor
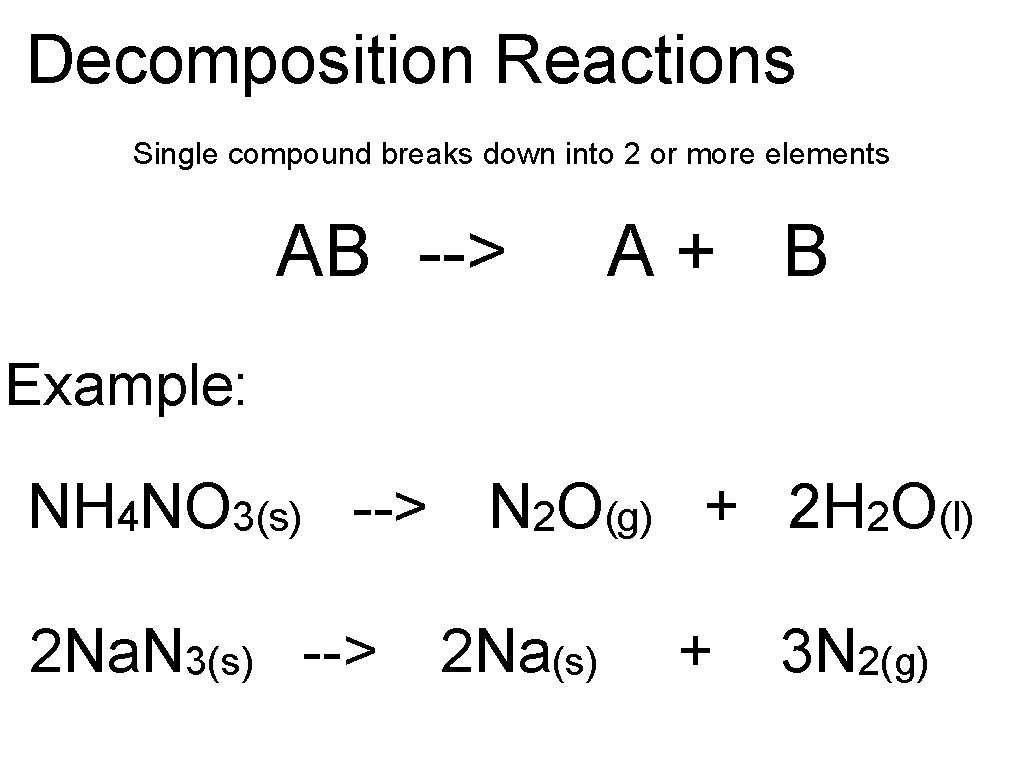
Decomposition Reactions Single compound breaks down into 2 or more elements AB --> A+ B Example: NH 4 NO 3(s) --> N 2 O(g) + 2 H 2 O(l) 2 Na. N 3(s) --> 2 Na(s) + 3 N 2(g)

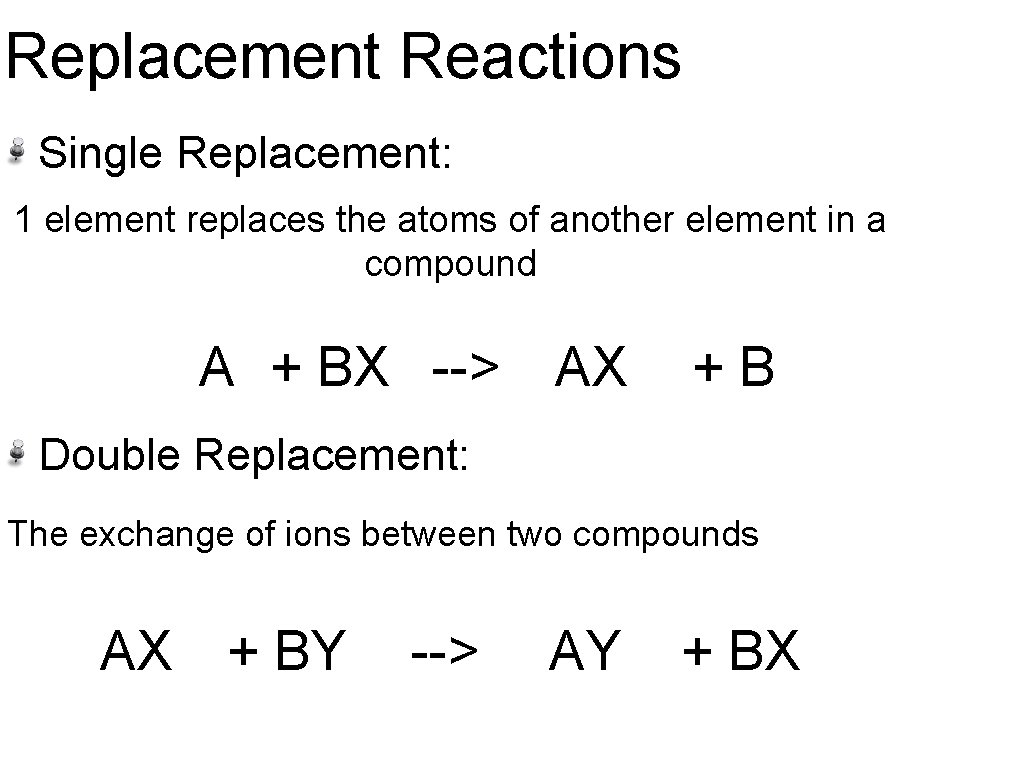
Replacement Reactions Single Replacement: 1 element replaces the atoms of another element in a compound A + BX --> AX +B Double Replacement: The exchange of ions between two compounds AX + BY --> AY + BX

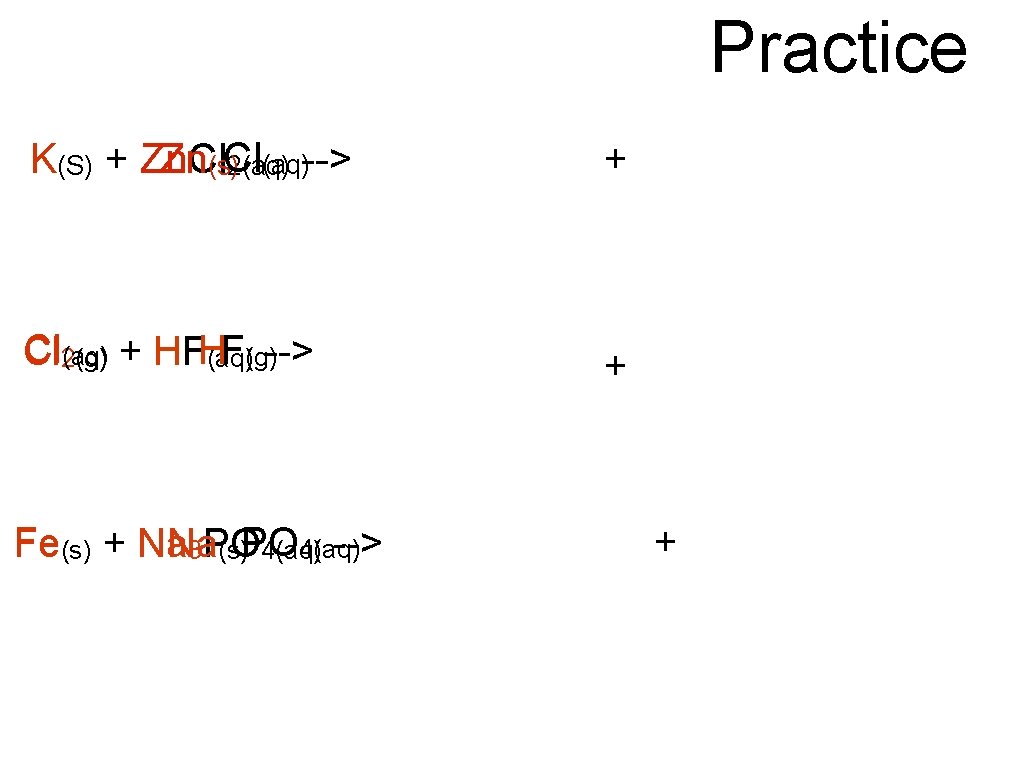
Practice K(S) + Zn. Cl Zn(s)Cl (aq)--> 2(aq) + Cl 2(g) (aq) + HFHF (aq)(g)--> + Fe(s) + Na O 4(aq) Na --> 3 PO (s)P 4(aq) +

AX + BY --> AY + BX Practice Aqueous lithium iodide and aqueous silver nitrate react to produce solid silver iodide and aqueous lithium nitrate.

AX + BY --> AY + BX Practice Aqueous barium chloride and aqueous potassium carbonate react to produce solid barium carbonate and aqueous potassium chloride.

Practice Aqueous sodium oxalate and aqueous lead(II) nitrate react to produce solid lead(II) oxalate and aqueous sodium nitrate.
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Combination reaction equation
Combination reaction equation Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Section 2 chemical reactions answer key
Section 2 chemical reactions answer key What is released or absorbed whenever chemical
What is released or absorbed whenever chemical























